Myotonic Dystrophy Treatment: In Search of Effective Weaponry to Kick Out Toxic RNAs Weakening Muscles
Dec 12, 2022
Myotonic dystrophy (DM), known as “the most variable of all diseases found in medicine,” is a multi-systemic genetic disorder that is the most common form of adult-onset muscular dystrophy. myotonic dystrophy is the only type of muscular dystrophy that affects cognition and brain function, in addition to the heart, lungs, muscles, gastrointestinal system, and many other organs.
Steinert, Batten, and Gibb identified myotonic dystrophy as a rare progressive disorder that is characterized by weakness, especially in muscles. It is also characterized by alterations in the central nervous system. There are two major types of myotonic dystrophy—Steinert disease or myotonic dystrophy type 1 (DM1) and a milder form, myotonic dystrophy type 2 (DM2) or proximal myotonic myopathy. Despite the overlap, the myotonic dystrophy symptoms of the disease vary from person to person and even among the subtypes of myotonic dystrophy. These constellations of symptoms make myotonic dystrophy a multifaceted and complex disorder. Currently, no myotonic dystrophy treatments are available to either delay or slow the progression of this disease.
Myotonic dystrophy is more common in European ancestry. Within DM, myotonic dystrophy type 1 is more common than myotonic dystrophy type 2 globally. Still, its prevalence varies widely from region to region. In the United States, roughly 26K patients develop myotonic dystrophy, with approximately 19K myotonic dystrophy type 1 cases. In Germany and Finland, myotonic dystrophy type 2 is more common than myotonic dystrophy type 1, accounting for nearly 5K cases versus 3K cases of myotonic dystrophy type 1 in 2019. As many individuals go undiagnosed, the true prevalence remains unknown among the general population.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Syndax Pharmaceuticals Receives FDA Approval for REVUFORJ; FDA Approves BLENREP for Adults with R...
- Merck’s KEYTRUDA as Adjuvant Therapy for RCC Patients; BMS Receives Positive CHMP Opinion for CAR...
- FDA Approves LEQEMBI IV Dosing for Early Alzheimer’s; Vanda Accepts FDA Hearing on Tradipitant fo...
As per the assessment done by DelveInsight, the total diagnosed prevalent patient population of myotonic dystrophy in the 7MM countries was close to 80K cases in 2021. As per the estimates, the US had the highest patient population of myotonic dystrophy in 2021.
The Myotonic Dystrophy Treatment Market is all set to Grow Significantly
Despite several pre-clinical developments, there is currently no specific disease-modifying myotonic dystrophy therapy available. Management entails primarily monitoring for complications and maintaining the standard of care (assistive devices, hormone therapy, and pain medication). A few myotonic dystrophy clinical trials have thoroughly examined the use of therapeutic agents. The lack of scientific evidence, combined with the disease’s multi-systematic and highly variable presentation, makes identifying and selecting appropriate medications especially difficult for prescribing physicians.
Further, as symptoms of many disorders can be similar to myotonic dystrophy, diagnosis can be difficult. According to the Christopher Project, a research collaboration to capture the diverse experiences of people living with myotonic dystrophy suggest uncertainty in myotonic dystrophy patients. Seventy per cent (70%) of respondents acknowledged they had received genetic confirmation of their disease, whereas 28% of patient respondents stated that they were unsure of the ‘type’ of myotonic dystrophy. Furthermore, the current market is captivated by symptomatic pharmacological interventions, which accounted for nearly USD 70 million in 2019 among the seven major markets.
As per DelveInsight analysis, the myotonic dystrophy market size was approximately USD 80 million in the 7MM in 2021, with the US accounting for the highest myotonic dystrophy market size than the EU5 (the United Kingdom, Germany, Italy, France, and Spain) and Japan.
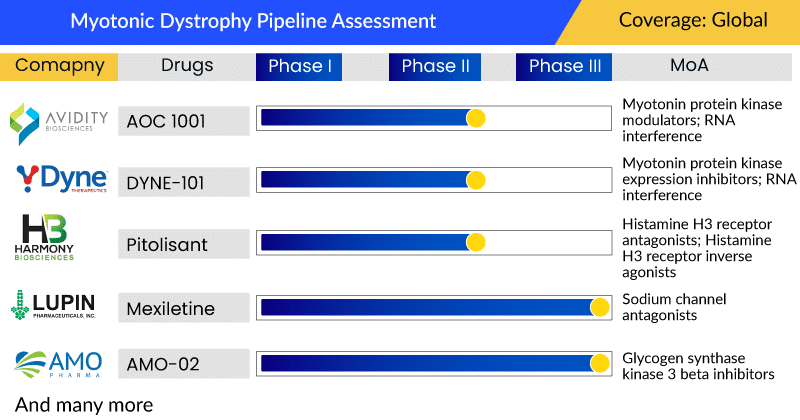
Moreover, with the advent of a few myotonic dystrophy pipeline candidates in late-stage development, targeting a specific pool of congenital myotonic dystrophy type 1 patients (AMO-02-AMO Pharma), myotonic dystrophy type 1 (Pitolisant-Harmony Biosciences) and myotonic dystrophy (Mexiletine-Lupin) the myotonic dystrophy market size is expected to increase. The emerging myotonic dystrophy treatment market consists of GSK3β inhibitors, sodium channel blockers, and H3 receptor antagonists. The launch of AMO-02, Mexiletine, and Pitolisant myotonic dystrophy drugs would offer more options to myotonic dystrophy patients currently inaccessible to augmentation therapy. Furthermore, the other assets in the myotonic dystrophy pipeline are in the early phase of development, including AOC 1001 (Avidity Biosciences) and DYNE-101 (Dyne Therapeutics).
In the last few decades, many research collaborations have emerged, helping raise awareness and targeting the general public to gather, educate and engage them as a combined faction. Another such campaign, “#30 days of strength,” was initiated by the Muscular Dystrophy Association (MDA) in September (awareness month) across the United States. This campaign engaged families, clinicians, and researchers for research and funding. The MDA is sponsoring 23 research grants with a total of USD 6,153,716 for myotonic dystrophy type 1 and myotonic dystrophy type 2.
Further, deep diving into experimental strategies like “expanded stretch of DNA” can help improve the outlook of people with myotonic dystrophy. Myotonic dystrophy type 1 and myotonic dystrophy type 2 correspond as a cause of this stretch of DNA on chromosomes 19 and 3, respectively; this expanded DNA steer formation of web-like toxic RNA, immobilizing vital proteins and leading to their deactivation and other repercussions. To skirmish these web-like structures, antisense oligonucleotides and other small molecules may form a righteous armament. These molecules attract enzymes that destroy the toxic RNA or separate them from cellular proteins. Thus, different tactics are being researched and developed with a similar goal.
Early Genetic Testing Key to Improving Myotonic Dystrophy Diagnosis
Early genetic testing and thorough clinical evaluation are key to a strong and impactful myotonic dystrophy diagnosis. Thus, it is necessary to implement early prenatal and genetic testing to understand inheritance and provide proper counselling. Realizing its importance, genetic analysis had been catching up, particularly in the neuromuscular field. As per the statistics, newborn babies are being screened for more than 30 conditions for which treatments are available in the United States. However, pitfalls and hurdles exist which are needed to be observed. Further, consensus-based recommendations for congenital and childhood-onset myotonic dystrophy type 1 have been published, but their implementation is to be checked worldwide.
Nevertheless, several assistance and patient advocate programs aim to help the families and caretakers fighting this deliberating disease. Apart from this, finding noninvasive and simple-to-measure biomarkers along with implementing vigilant monitoring and pre-emptive measures could aid a lot in managing disease until a cure is found.

FAQs
Steinert, Batten, and Gibb identified myotonic dystrophy (DM) as a rare progressive disorder that is characterized by weakness, especially in muscles. It is also characterized by alterations in the central nervous system. There are two major types of myotonic dystrophy—Steinert disease or myotonic dystrophy type 1 (DM1) and a milder form, DM2 or proximal myotonic myopathy.
The myotonic dystrophy symptoms vary from person to person and even among the subtypes of myotonic dystrophy despite the overlap. These constellations of myotonic dystrophy symptoms make myotonic dystrophy a multifaceted and complex disorder.
A thorough clinical evaluation, a detailed patient and family history, and identifying characteristic physical findings may lead to a diagnosis of myotonic dystrophy. Several laboratory tests, including blood tests, electromyography (EMG), magnetic resonance imaging (MRI), muscle biopsy, and genetic testing, can help to clarify the clinical myotonic dystrophy diagnosis. Though various diagnostic options exist, the definitive test for myotonic dystrophy is a genetic test.
Currently, no approved treatment provides a permanent cure for myotonic dystrophy, but researchers are looking into ways to help people with these disorders. The current myotonic dystrophy treatment focuses on each individual’s specific symptoms. Pharmacological management, rehabilitative therapy, medical devices, and surgical procedures are all therapeutic options tailored to myotonic dystrophy symptoms’ severity.
Currently, leading companies such as AMO Pharma Limited (AMO-02), Lupin Ltd (Mexiletine), and Harmony Biosciences, LLC (Pitolisant) are developing therapies for myotonic dystrophy treatment. Furthermore, the other assets in the pipeline are in the early phase of development, including AOC 1001 (Avidity Biosciences) and DYNE-101 (Dyne Therapeutics).
Downloads
Article in PDF
Recent Articles
- Syndax Pharmaceuticals Receives FDA Approval for REVUFORJ; FDA Approves BLENREP for Adults with R...
- FDA Approves LEQEMBI IV Dosing for Early Alzheimer’s; Vanda Accepts FDA Hearing on Tradipitant fo...
- Merck’s KEYTRUDA as Adjuvant Therapy for RCC Patients; BMS Receives Positive CHMP Opinion for CAR...




