How Decisive Will The OTC Approval of NARCAN Be In The Fight Against The Opioid Epidemic?
May 15, 2023
On March 29, 2023, the FDA made the landmark decision to approve Emergent BioSolutions’ NARCAN nasal spray for over-the-counter (OTC) and nonprescription use, making it the first naloxone product to be approved for use without a prescription.
With about 80,000 fatal opioid overdoses every year, this could potentially come as a historic decision by the FDA in its continuous fight against the opioid crisis, as the exact impact of this decision is yet to be known. According to Emergent, the 4 mg nasal spray of naloxone is expected to be available in stores and online by late summer.
This decision aims to increase access to naloxone in the community setting. According to the CDC, nearly half of the opioid overdose deaths in 2021 had potential bystanders who could have prevented a death if NARCAN was available. Despite first responders having had access to naloxone for decades, it often takes several minutes for them to respond to an overdosing patient, which is crucial in deciding the patient’s survivability. This is why getting NARCAN in the hands of bystanders is vital.
Downloads
Article in PDF
Recent Articles
- 4D Molecular Therapeutics raises; Arvinas slates for IPO; Sutro ruminates for $ 75M; Emergent Bio...
- Tesaro’s Zejula; Mylan on EpiPen; Ohio sues drugmakers; ATUM, Horizon Discovery Announce deal
- Regeneron’s cocktail antibody, REGN-COV2; J&J’s manufacturing-spree; ObsEva̵...
- Emerging Therapies in the Chronic Pain Pipeline Transforming the Chronic Pain Market Landscape
- Opioid-Induced Respiratory Depression: Unveiling the Silent Threat

The economic barriers to naloxone access and a long-delayed federal response to the opioid crisis have led to the loss of thousands of lives. The failed “War on Drugs,” which criminalizes drugs and imprisons people who use them, helps explain the flaws of past policies that were overinvested in legal enforcement and underinvested in addiction treatment and harm reduction. Although in the past few years, new administrative policies have focused on a more compassionate, humanizing approach to the problem, where the importance of harm reduction and addiction treatment has been elevated.
In 2017, the US Health and Human Services (HHS) declared the opioid epidemic a public health emergency. Recently, the Biden Administration has nominated Rahul Gupta—former health commissioner of West Virginia—as head of the Office of National Drug Control Policy, the first physician to take on the role, signifying less focus on legal and law enforcement approaches to drug policy and an increased emphasis on addiction treatment and expanded healthcare services. Also, the rolling back of the X-wavier by the DHHS in April 2021 is set to enable more healthcare providers to prescribe buprenorphine for opioid use disorder treatment. These recent developments signify the US government’s shifting policies in its fight against the opioid epidemic.
The CDC categorizes opioids into four categories, which are:
Naloxone should always be given if someone is suspected of having an opioid overdose, as it does not harm the person if they overdose on something other than opioids. Moreover, Good Samaritan laws are in place in most states, protecting those overdosing and anyone assisting them in an emergency from arrest, charges, or a combination of these.
Naloxone was first approved in the United States in 1971 with the trade name NARCAN, originally approved as an injectable formulation that could be administered intravenously (IV), intramuscularly (IM), or subcutaneously (SC). Both medical staff and first responders used it extensively.
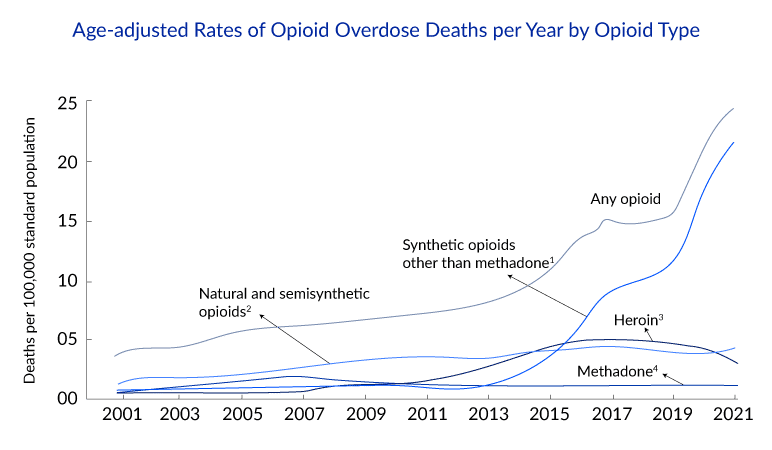
But, with increasing opioid abuse and overdoses, the prominence of the use of naloxone in the non-healthcare setting increased. Multiple initiatives across the United States provided naloxone and instructions for its use to populations at risk of opioid overdose and their family, friends, and/or caregivers. These programs were effective at getting naloxone into the community setting.
However, because the injectable naloxone products at the time required additional preparation or assembly before administration, there was a public health need for naloxone products that did not require additional preparation or assembly before administration and could be administered quickly and safely by a layperson.
Since 2014, FDA has approved several prescription naloxone drug-device combination products for the emergency treatment of a known or suspected opioid overdose, which were approved explicitly with the intention of “community use.” “Community-use” products facilitate laypersons’ use without additional supplies or assembly before use.
The following are approved products specifically designed for layperson use:
Based on FDA’s analyses, nationally estimated sales and dispensed prescriptions for naloxone products increased across all healthcare settings from 2017 to 2021, largely due to a substantial increase in naloxone nasal spray distribution.
The estimated number of naloxone units sold increased by 81% from approximately 5.1 million units in 2017 to about 9.3 million units in 2021.
Injectable and nasal spray sales to hospitals increased by more than 50%, and sales to retail pharmacies tripled during this period. In 2017, approximately half of naloxone sales to retail pharmacies were for the nasal spray; by 2021, over 90% were for the nasal spray. Similar to sales to healthcare settings, the estimated number of naloxone prescriptions dispensed from pharmacies increased from under half a million prescriptions in 2017 to 1.5 million prescriptions in 2021. In 2021, over 95% of naloxone prescriptions dispensed from US outpatient retail, mail-order, and long-term care pharmacies were for the nasal spray.
Although it is to note that these estimates do not consider the availability and distribution of naloxone products due to donations from manufacturers and direct sales to community-based naloxone distribution programs, accounting for these could increase the distribution and availability of naloxone products significantly, as according to some sources, community-based naloxone distribution programs received over 2 million injectable naloxone doses donated by manufacturers or purchased in bulk at low cost between 2017 and 2021.
Although this decision of OTC naloxone approval marks a huge step in the fight against the opioid crisis, it came significantly later than it should have. At a conference in 2017, FDA officials asked naloxone manufacturers to apply for OTC status. This was specifically aimed at two naloxone products that had been then approved for layperson use; one was EVZIO, an auto-injector modeled after the EpiPen, made by Kaléo, and the other was NARCAN Nasal Spray, made by ADAPt Pharma, which Emergent BioSolutions have since acquired.
The FDA even took the unprecedented step of creating a Drug Facts Label for naloxone and published the methodology for the label in the New England Journal of Medicine. Such labels are typically the responsibility of companies, which are required for OTC submission, and developing and testing them takes a lot of time and resources.
Despite the steps the FDA took to provide an easy path for companies to apply for OTC status of naloxone products, the companies made a conscious decision not to step forward. This was the case as companies manufacturing naloxone products charge a significant premium on their products since they get reimbursed for it as a prescription, significantly contributing to their revenues. Hence, looking at the situation from an economic standpoint, making these products available over the counter could substantially hamper their revenues.
EVZIO was the first auto-injector designed to deliver a dose of naloxone outside of a healthcare setting. It was later discontinued in 2019 by Kaléo for undisclosed reasons, but it came after a 2018 congressional investigation into Kaléo’s decision to hike EVZIO’s price from USD 575 per unit to USD 4,100. The company subsequently announced it would make a generic version available at USD 178 per unit, then discontinued the generic and brand-name versions due to a lack of profitability.
Even before the OTC approval of NARCAN, nearly every state in the US has had standing orders that allowed pharmacists or other qualified organizations to provide the medication without a personal prescription to people who are at risk of an overdose or are helping someone at risk, but making it available over the counter can make it easier for people to access the opioid antidote.
NARCAN Nasal Spray was developed for use in the community setting, and more than 44 million doses have been distributed since its launch in 2016. With the entry of its generics in December 2021, NARCAN sales dropped from USD 434.3 million in 2021 to USD 373.3 million in 2022. Driven by this decline in revenue and potential competition from Harm Reduction Therapeutics’ RiVive, Emergent filed and got the approval for NARCAN Nasal Spray’s OTC availability.
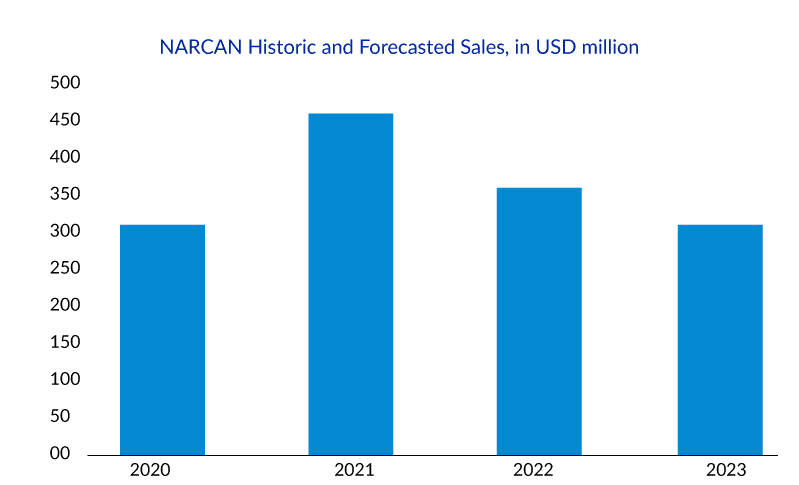
Harm Reduction Therapeutics is a nonprofit pharmaceutical company funded by Purdue Pharma, focusing on improving access and availability of naloxone. RiVive is their 3 mg nasal spray of naloxone, currently under priority review by the FDA, expected to be completed by the summer of 2023. If RiVive obtains approval by then, it is expected to be available by early 2024 at a meager cost, if not for free.
If RiVive succeeds and enters the market, it will be a systemic fix in the fight against the opioid crisis, like a systemic therapy for the entire US public health system in its fight against the epidemic.
Putting it on the shelves will allow people to pick it up without dealing with the stigma attached. Despite the positive outlook being seen by people on the social change brought about by this decision, in helping get past the stigma, a driver of the overdose crisis, the concern remains on how NARCAN will be priced after its OTC availability and how insurance coverage will work on it. It would be a terrible shame if one barrier to access were removed only to have another take its place.
Currently, buyers have to pay for NARCAN with an insurance co-pay or for the full retail price, which varies, with two doses of NARCAN being priced at about USD 120.
Recently on April 20, 2023, Emergent announced plans to price NARCAN at less than USD 50 for two doses. Despite the fact that this price is much lower than the current list price, healthcare providers argue it is still too high, especially for people with opioid addiction, who often live below the federal poverty line.
According to harm-reduction experts, the high price of naloxone has historically inhibited its access to people who need it the most. And despite the lowering of price after OTC availability, the cost would still be high enough to remain out of reach for many.
The US Centers for Medicare and Medicaid Services, which now cover prescription naloxone for people on government insurance programs, says that coverage of over-the-counter naloxone would depend on the insurance program. The centers have not given any official guidance.
Although lowering the drug’s cost would improve NARCAN’s availability, it could, in turn, affect its profitability, forcing the drug maker to stop its production, as was the case with EVZIO. But, as per experts of Public Health, the approval of the first over-the-counter product will encourage other pharmaceutical companies to develop newer and cheaper formulations. Hence competition is set to bring innovation, which in turn would end up saving lives.
Hence, a steady balance between availability and profitability must be maintained while deciding a fair price for NARCAN.
This constant balance in a slippery slope that companies have to maintain between accessibility and profit sheds light on the possible role of nonprofits, where companies like Harm Reduction Therapeutics could ensure the wide availability of over-the-counter naloxone without worrying about profits.
According to experts, the most meaningful work in the fight against the devastating outcomes of the drug overdose epidemic will come with an ongoing emphasis on opioid use disorder treatment and other harm-reduction strategies.
While enabling people to access quality opioid use disorder treatment is critical, the need for people to survive in order to have that choice should also be acknowledged. Hence, for an opioid-dependent person who experiences an overdose, which is then controlled by naloxone, it could act as a ‘gateway drug’ for treating their opioid use disorder.
Opioid use disorder treatment with opioid agonists like methadone or buprenorphine is a game-changing approach toward preventing opioid overdoses, leading to a population-level impact in the fight against the opioid epidemic.
Other than the opioid agonists, the opioid antagonist naltrexone is also used frequently for opioid use disorder treatment. Although it should be noted that, despite both naloxone and naltrexone being opioid antagonists, they differ very much in how they are used. Naloxone is a short-acting drug used to reverse the effects of an opioid overdose, while naltrexone is used as a long-acting opioid use disorder treatment.
FAQs
Opioid use disorder is defined as the prolonged use of opioids that causes clinically substantial distress or impairment. Opioid use disorder is characterized by an overwhelming desire to use opioids, increased opioid tolerance, and withdrawal symptoms when opioids are withdrawn.
The opioid use disorder symptoms of OUD can vary depending on the severity of the condition. Some common opioid use disorder symptoms include strong cravings for opioids, tolerance, withdrawal symptoms, continued use despite negative consequences, difficulty controlling use, neglecting responsibilities, using opioids in risky situations, mood changes, and others.
Diagnosing opioid use disorder involves a comprehensive evaluation of an individual’s symptoms, medical history, and drug use patterns. The diagnostic criteria for OUD are outlined in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), which is the standard reference used by healthcare professionals to diagnose mental health conditions.
There are several effective treatment options available to individuals struggling with this condition. Medication-assisted treatment (MAT) is the most commonly recommended approach and involves the use of medications such as methadone, buprenorphine, or naltrexone, in conjunction with behavioral therapy. These medications help to reduce cravings and withdrawal symptoms, allowing individuals to focus on their recovery and achieve long-term sobriety. Other forms of therapy, including cognitive-behavioral therapy, individual and group counseling, and peer support groups, can also be helpful in treating opioid use disorder. Successful treatment often involves a comprehensive approach that addresses the physical, emotional, and social aspects of addiction and provides ongoing support to individuals in recovery.

Downloads
Article in PDF
Recent Articles
- Emerging Therapies in the Chronic Pain Pipeline Transforming the Chronic Pain Market Landscape
- CTEXLI Approved for Cerebrotendinous Xanthomatosis; SIGX1094 Wins Fast Track for Diffuse Gastric ...
- Tesaro’s Zejula; Mylan on EpiPen; Ohio sues drugmakers; ATUM, Horizon Discovery Announce deal
- Opioid-Induced Respiratory Depression: Unveiling the Silent Threat
- 4D Molecular Therapeutics raises; Arvinas slates for IPO; Sutro ruminates for $ 75M; Emergent Bio...