Advances in NASH Therapeutics Space: Latest Breakthroughs in Drug Development
Oct 09, 2024
Table of Contents
Nonalcoholic steatohepatitis (NASH), also referred to as Metabolic Dysfunction-Associated Steatohepatitis (MASH), has rapidly become a significant contributor to liver disease in the United States. The NASH market is growing at a substantial rate, driven by the increasing prevalence of the condition. According to the National Institute of Diabetes and Digestive and Kidney Diseases, while nonalcoholic fatty liver disease (NAFLD) remains common, only a fraction of these patients progress to NASH. As of 2023, the U.S. had the highest number of diagnosed NASH prevalent cases, with approximately 9.7 million individuals affected. Following closely behind were the EU4 nations and the UK, with around 3.8 million cases, and Japan, which reported about 2.6 million cases.
Interestingly, a gender-based analysis reveals that men are disproportionately affected by the disease, comprising 56% of all cases across the 7 major markets (7MM). Japan, in particular, accounted for approximately 16% of all NASH cases in 2023, a figure that is projected to rise as the overall prevalence continues to increase between 2020 and 2034.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Lilly’s Morphic Acquisition; IDEAYA’s IDE397 Positive Phase II Trial Result; XPOVIO (seline...
- Good news for Kite’s KTE-X19, and Aquestive’s Libervant; and a commercialization deal between ReF...
- Sanofi’s Qfitlia Becomes First FDA-Approved Therapy for Hemophilia A or B; FDA Approves AstraZene...
- STAT INHIBITORS- Highly active Pipeline
- Business Cocktail
This surging demand underscores the urgent need for effective NASH therapies and highlights the untapped potential for innovative NASH drugs. As the condition continues to gain global attention, the search for the next breakthrough NASH therapy is intensifying, creating lucrative opportunities for pharmaceutical companies aiming to target this widespread liver disease.
REZDIFFRA: The First-Ever Drug Approval for NASH
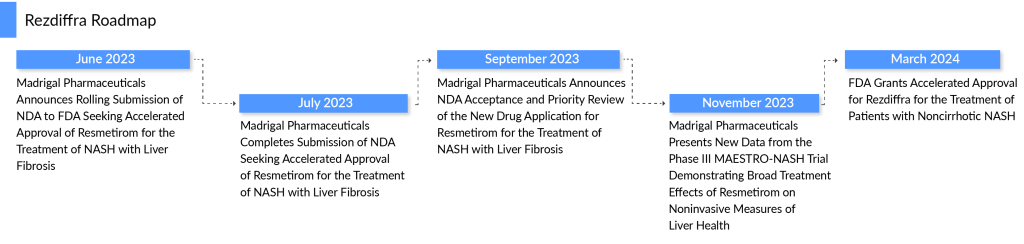
In March 2024, REZDIFFRA (resmetirom) made history by becoming the first drug approved by the FDA for treating NASH with moderate to advanced liver fibrosis (F2 to F3 stages). Developed by Madrigal Pharmaceuticals, this thyroid hormone receptor-beta (THR-β) agonist targets the root causes of NASH, making it a breakthrough in addressing liver fibrosis and fat accumulation in affected patients.
The drug, which received breakthrough therapy, fast-track, and priority review designations, is available in multiple dosages, including 60mg, 80mg, and 100mg. REZDIFFRA’s approval is a significant milestone in NASH therapy, offering new hope to patients struggling with the disease’s progressive and potentially life-threatening impact on liver health.
Uncover the breakthrough in NASH treatment—read our article on REZDIFFRA!
In the latter part of September 2024, Madrigal Pharmaceuticals continued its preparations for the launch of its pioneering NASH treatment, resmetirom, following the FDA’s acceptance of its New Drug Application (NDA) and priority review.
Madrigal also raised $500 million in a public offering, on top of the $309 million raised in December 2022, following positive results from the pivotal Phase III MAESTRO-NASH trial. While the company has solid financial backing, analysts note that Madrigal may seek a partnership with a larger pharmaceutical company or even an acquisition to ensure a successful market launch for resmetirom, the first-ever NASH drug.
Dive into the trailblazing journey of REZDIFFRA in transforming NASH care—check it out now!
Navigating the NASH Treatment Landscape: Recent Breakthroughs and Trials
Now, like many other diseases, NASH has seen numerous clinical trials over the years. Each trial offers critical insights into potential treatments and brings us closer to discovering the next blockbuster NASH drug. These trials become even more vital in a field where few approved treatments exist. While a cure may still be out of reach, the ongoing search for effective treatment plans is well underway.
So, let’s dive into the NASH treatment landscape and explore the latest commercial developments over the past few months. Has a drug finally been approved to combat this silent menace?
August 2024
Shilpa Medicare’s SMLNUD07 Demonstrates Success
In August 2024, Shilpa Medicare experienced a stock surge following the successful Phase III trials of SMLNUD07 (NorUDCA) in treating NAFLD. This drug, now showing promise in addressing liver fibrosis and fat accumulation, was tested across India, enrolling 165 patients. The trial yielded significant results, showing at least one stage of liver fibrosis improvement without serious adverse events.
Shilpa Medicare’s drug is expected to revolutionize NAFLD treatment, with potential to become a new standard of care. Investors responded positively, viewing SMLNUD07 as a pivotal drug for patients suffering from liver disease, further strengthening Shilpa’s position in the market.
April 2024
D&D Pharmatech Receives Fast Track Designation for DD01
In April, D&D Pharmatech announced that its investigational drug, DD01, received Fast Track designation from the FDA for treating NASH. This designation helps expedite the development and review process, potentially bringing this promising drug to market sooner. DD01 has already shown efficacy in reducing liver fat by over 50% in clinical studies, with some patients achieving as much as a 100% reduction in liver fat content.
With these impressive results, DD01 could represent a significant advancement in NASH treatment, addressing the metabolic complications of the disease alongside liver health. The Fast Track designation signals FDA confidence in DD01’s potential to meet the unmet medical needs of NASH patients.
March 2024
Inventiva Resumes NATiV3 Trial After Voluntary Pause
In March 2024, Inventiva announced the resumption of its NATiV3 clinical trial, following a temporary voluntary pause due to a suspected adverse reaction in one of the trial’s patients. The NATiV3 trial is focused on evaluating the safety and efficacy of lanifibranor in NASH patients. The pause allowed for amendments to the trial protocol and patient screening processes, including more stringent liver monitoring protocols.
The resumption of this trial marks a crucial step forward for Inventiva, as lanifibranor continues to show potential in addressing liver fibrosis and inflammation in NASH patients. The company anticipates completing the trial by the first half of 2024, which could pave the way for further development or regulatory submission based on the results.
February 2024
Zealand Pharma’s Survodutide Hits Phase II Milestones
In February 2024, Zealand Pharma and Boehringer Ingelheim announced exciting results from the Phase II trial of survodutide in treating MASH. Survodutide, a glucagon/GLP-1 receptor dual agonist, showed 83% efficacy in reducing liver fibrosis and metabolic dysfunction, significantly surpassing the placebo group’s 18.2% response rate. This drug is aimed at treating people with obesity who also suffer from MASH, a demographic with a high unmet need.
The success of survodutide in MASH further solidifies its potential to become a best-in-class treatment. Survodutide has already shown efficacy in cardiovascular and renal conditions, adding to its potential multi-faceted benefits. These results, alongside the ongoing Phase III trial, could lead to a fast-track toward approval, marking another significant stride in the fight against NASH.
January 2024
Merck & Co’s Focus on GLP-1 Class for NASH Treatment
In early 2024, Merck & Co. made a strategic move by entering the non-alcoholic steatohepatitis (NASH) treatment space with its experimental GLP-1 class drug, efinopegdutide. This drug is designed to go beyond traditional weight-loss treatments, targeting metabolic dysfunction and showing a promising weight-loss benefit. GLP-1 drugs, which help control blood sugar and induce a feeling of fullness, have gained immense popularity due to their potential to impact broader health outcomes, including cardiovascular and metabolic disorders.
According to Merck’s CEO, Robert Davis, this GLP-1 class treatment for NASH represents an opportunity to enter a market where newer drugs like WEGOVY and OZEMPIC are already generating significant traction. In light of data suggesting that the active ingredients in these drugs may reduce the risk of stroke, heart attacks, and kidney disease, Merck’s efinopegdutide could carve out a substantial place in the NASH landscape by addressing weight loss and liver health simultaneously. The company’s efforts come when competition in the GLP-1 space is intensifying, with other players like Novo Nordisk and Eli Lilly exploring similar avenues.
Boston Pharmaceuticals Advances BOS-580 for NASH
In January 2024, Boston Pharmaceuticals presented promising data on its long-acting fibroblast growth factor 21 (FGF21) analogue, BOS-580, at the NASH-TAG 2024 conference. The data suggested low immunogenicity and positive clinical effects in metabolic dysfunction-associated steatohepatitis (MASH). The results were particularly significant because patients need sustained efficacy over time without adverse immunological reactions, a common concern with other FGF21 analogues.
The company highlighted BOS-580’s impact on several non-invasive markers of liver injury and fibrosis in patients. CEO Sophie Kornowski emphasized that this low immunogenicity could help BOS-580 stand out from other FGF21 analogues currently in development. Boston Pharmaceuticals aims to advance this once-monthly treatment into pivotal clinical trials soon, positioning BOS-580 as a potential game-changer in NASH care. The combination of efficacy and convenience may offer patients a compelling new treatment option in a landscape with limited effective therapies.
Sagimet Biosciences Shows Promise with Denifanstat
Also in January, Sagimet Biosciences made headlines with positive topline results from its FASCINATE-2 Phase IIb clinical trial of denifanstat, a selective fatty acid synthase (FASN) inhibitor. The trial, which included biopsy-confirmed NASH patients with stage 2 or 3 fibrosis, showed significant improvements in NASH resolution and reductions in fibrosis compared to placebo. Specifically, 36% of patients treated with denifanstat achieved NASH resolution without worsening of fibrosis, while 52% saw at least a 2-point reduction in their NAFLD Activity Score (NAS), making denifanstat a strong contender for NASH treatment.
Additionally, denifanstat demonstrated strong secondary endpoints, with 41% of patients showing fibrosis improvement by at least one stage without worsening NASH. The drug also achieved remarkable results in MRI-based assessments of liver fat reduction. With these results, Sagimet is preparing to move forward with further development, anticipating that denifanstat could become a key player in the NASH market.
What’s Next in the NASH Treatment Landscape?
While REZDIFFRA has cemented its place as the first-ever approved therapy for NASH, another promising contender is on the horizon. In June 2023, Zydus Lifesciences initiated a Phase-IV trial for Saroglitazar Mg in patients with NAFLD. The company is evaluating the use of its drug, Lipaglyn, to treat liver conditions like NASH, NAFLD, and Primary Biliary Cholangitis. The earlier EVIDENCES IV trial conducted in the U.S. showed impressive results, with Saroglitazar Mg achieving the primary endpoint by reducing alanine aminotransferase levels by 44.39% over 16 weeks. Beyond that, the drug showed significant improvements in liver fat content, insulin resistance, and dyslipidemia.
In the ongoing Evidences-XI trial, around 1,500 male and female NAFLD patients with comorbidities are enrolled. Preliminary findings suggest that Saroglitazar Mg could improve liver enzymes, reduce liver stiffness, and enhance metabolic parameters in both NAFLD and NASH patients. Currently, Saroglitazar Mg is only approved in India, where it is prescribed for hypertriglyceridemia, diabetic dyslipidemia, and NASH with comorbidities.
In the U.S., Saroglitazar Mg is still an investigational drug undergoing Phase II trials for NASH treatment. Additionally, Zydus has completed the Phase IIb/III EPICS III trial for PBC. The drug has received Orphan Drug Designation and Fast Track Designation from the FDA for PBC and has also been granted Orphan Status by the EMA.
If the trials continue to show positive results, Saroglitazar Mg could very well be the next big player in the NASH treatment market.
In addition to this, several other prominent players are actively involved in the development of NASH treatments. These include Galmed Research and Development, Ltd., Galectin Therapeutics, Rivus Pharmaceuticals, Lipocine, Enyo Pharma, HighTide Biopharma, Sagimet Biosciences, Poxel SA, NGM Biopharmaceuticals, Boston Pharmaceuticals, Pfizer, AstraZeneca, Novo Nordisk A/S, Kowa Research Institute, Gilead Sciences/Novo Nordisk A/S, Inventiva Pharma, Cirius Therapeutics, 89bio, Akero Therapeutics, CytoDyn, NorthSea Therapeutics BV, Hepion Pharmaceuticals, and many others.
Conclusion
As the battle against Non-Alcoholic Steatohepatitis heats up, the landscape is rapidly evolving with promising developments that offer hope for millions suffering from this silent yet formidable adversary. With the groundbreaking approval of REZDIFFRA marking a historic milestone in NASH treatment, the spotlight now shines on many innovative therapies that are on the cusp of redefining the NASH market. From Merck’s cutting-edge GLP-1 class drug to Boston Pharmaceuticals’ game-changing BOS-580, the relentless pursuit of effective NASH therapies is invigorating the NASH arena.
And let’s not forget the rising star Saroglitazar Mg, whose potential could disrupt market dynamics if trials continue to deliver encouraging results. With NASH prevalence on the rise and industry giants and nimble innovators alike rallying together, they are poised to create a wave of advancements that could transform the lives of those grappling with liver disease. With every clinical trial, breakthrough, and strategic partnership, we inch closer to a future where NASH drugs are not just managing the condition but conquering it.
The fight is far from over, but the spirit of innovation is alive and well, ready to usher in a new era of hope for patients everywhere. Buckle up—this journey is just starting, and the best is yet to come!

Downloads
Article in PDF
Recent Articles
- Eisai Submits Marketing Authorization Application for Tasurgratinib; CHMP Issues Positive Opinion...
- AstraZeneca’s COVID-19 vaccine; Type 1 diabetes research updates; FDA nod to Zebra’s X-ray ...
- What lies in the future for NASH upcoming drugs?
- FDA Approves PENBRAYA for Most Common Serogroups Causing Meningococcal Disease; BIMZELX Approved ...
- DelveInsight launches Indication Active Pharmaceutical Ingredient (API) Reports



