NASH Emerging Therapies: The Path Forward
Sep 04, 2024
Table of Contents
If you’ve been keeping up with our previous posts, you already have a solid understanding of nonalcoholic steatohepatitis (NASH) and its impact. We’ve previously examined the dominance of off-label therapies in the NASH therapeutics market and the challenges associated with this approach. In this post, we’ll take a deeper dive into the future of NASH treatments, exploring emerging therapies and innovations that could reshape the landscape in the years to come.
In 2024, the world witnessed a significant milestone with the approval of REZDIFFRA (resmetirom), the first drug specifically designed to treat nonalcoholic steatohepatitis (NASH) in adults. NASH, a severe form of fatty liver disease, can progress to liver scarring, dysfunction, cirrhosis, and ultimately liver failure. REZDIFFRA, a daily oral medication, works by activating a thyroid hormone receptor to reduce liver fat accumulation. The FDA granted its approval for adults with moderate to advanced liver scarring, to be used alongside diet and exercise.
Downloads
Article in PDF
Recent Articles
- Nonalcoholic Steatohepatitis (NASH): A Growing Epidemic
- Redefining Liver Disease in the Metabolic Era: From NASH to MASH and the Rise of Obesity‐targeted...
- TLX66 in AL Amyloidosis; Takeda’s Dengue Shot; Innovent’s Cholangiocarcinoma Clinical Trial; Anot...
- Empowering Change: REZDIFFRA’s Trailblazing Journey in NASH Treatment
- Notizia
Approved under the FDA’s accelerated approval pathway, which expedites the availability of treatments for serious conditions, REZDIFFRA represents a major breakthrough in liver health. Its approval is seen as a pivotal moment that could pave the way for additional NASH therapies. Given the immense unmet need for effective treatments for this widespread and devastating disease, researchers and scientists are actively pursuing therapies that specifically target NASH. According to estimates from DelveInsight, several promising drugs are expected to be launched in the coming years. Key players in the NASH market include Inventiva Pharma, Zydus Therapeutics, Novo Nordisk A/S, Eli Lilly and Company, Madrigal Pharmaceuticals, Inc., Terns, Inc., Intercept Pharmaceuticals, Enyo Pharma, 89bio, Inc., Akero Therapeutics, Inc., and others.
In 2023, prominent liver disease organizations, including the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL), officially redefined Nonalcoholic Steatohepatitis (NASH) to Metabolic Dysfunction-Associated Steatohepatitis (MASH), aligning the name more closely with its metabolic roots. For the most up-to-date insights, explore our comprehensive MASH Market Report.
Emerging Therapies in the NASH Pipeline
With the rising prevalence of nonalcoholic steatohepatitis and the severe complications it can lead to, the need for effective treatments has never been more critical. While the development of new therapeutics for NASH has faced numerous challenges, recent advancements have brought promising candidates to the forefront. As we look ahead, several emerging therapies are poised to potentially revolutionize NASH treatment, offering hope to millions affected by this condition. Here’s a closer look at some key drugs in the pipeline.
Lanifibranor: Inventiva Pharma
Lanifibranor, Inventiva’s lead product candidate, is an orally-available small molecule that acts to induce antifibrotic, anti-inflammatory and beneficial vascular and metabolic changes in the body by activating all three peroxisome proliferator-activated receptor (PPAR) isoforms, which are well-characterized nuclear receptor proteins that regulate gene expression. Lanifibranor is a PPAR agonist that is designed to target all three PPAR isoforms in a moderately potent manner, with a well-balanced activation of PPARα and PPARδ, and a partial activation of PPARγ. While other PPAR agonists target only one or two PPAR isoforms for activation. The FDA has granted Breakthrough Therapy and Fast Track designation to lanifibranor for the treatment of NASH. Currently, the drug is in Phase III stage of its clinical trial for the treatment of NASH.
MSDC-0602K: Cirius Therapeutics
MSDC-0602K, a second-generation oral insulin sensitizer, is designed to selectively modulate the mitochondrial pyruvate carrier (MPC) while minimizing direct PPAR-gamma activation. The MPC mediates at the cellular level the effects of over nutrition, a major cause of Nonalcoholic fatty liver disease NAFLD/NASH and Type 2 diabetes. In preclinical studies, modulation of the MPC has been shown to improve insulin sensitivity, lipid metabolism, and inflammation. Currently the drug is in Phase III stage of Clinical trial for the treatment of NASH.
TERN-501: Terns Pharmaceuticals
TERN-501 is a THR-β agonist with high metabolic stability, enhanced liver distribution and greater selectivity for THR-β compared to other THR-β agonists in development. Agonism of THR-β increases fatty acid metabolism via mitochondrial oxidation and affects cholesterol synthesis and metabolism. As a result, THR-β stimulation has the ability to reduce hepatic steatosis and improve serum lipid parameters including LDL cholesterol and triglycerides. In vivo NASH studies in a rodent model have demonstrated that low-doses of TERN-501 achieved complete resolution of steatosis and reductions in serum lipids, hepatic inflammation and fibrosis. TERN-501 has high liver distribution and is 23-fold more selective for THR-β than for THR-α activation in a cell free assay, thereby minimizing the risk of cardiotoxicity and other off-target effects associated with non-selective THR stimulation. Currently, the drug is in Phase II stage of its clinical trial for the treatment of NASH.
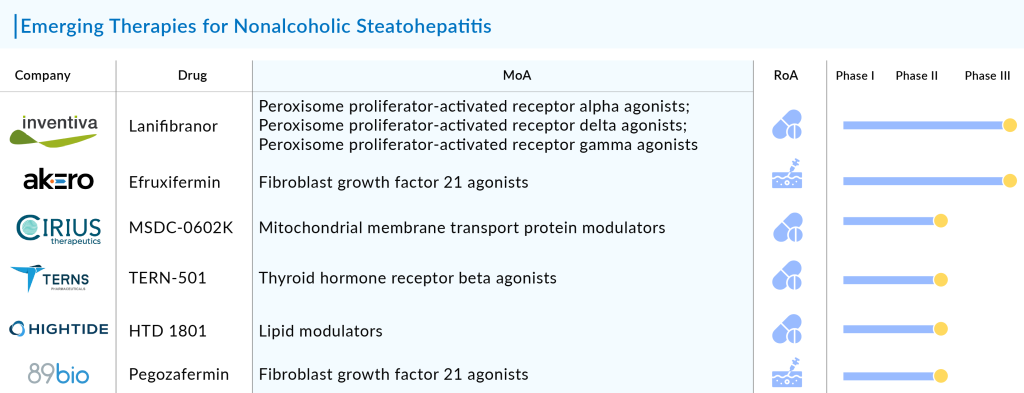
HTD 1801: HighTide Biopharma
The company’s lead drug candidate, HTD1801, is a first-in-class new molecular entity (ionic salt of two active moieties). It is a novel orally active ionic salt of berberine and ursodeoxycholic acid, substantially reduced liver fat while improving glycemic control and other cardiometabolic biomarkers in adults with nonalcoholic steatohepatitis (NASH) and type 2 diabetes (T2DM). Currently, it is in Phase II trials for the treatment of primary sclerosing cholangitis (PSC), and nonalcoholic steatohepatitis (NASH).
Thiazolidinediones (TZD): Pioglitazone and Rosiglitazone
TZDs, including pioglitazone and rosiglitazone, are PPARγ agonists primarily located in adipose tissue, where they play a crucial role in regulating adipocyte differentiation, adipogenesis, and lipid metabolism. They have been shown to improve glucose uptake, increase fatty acid oxidation, and enhance insulin secretion, leading to improved insulin sensitivity. A novel dual GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 receptor agonist. In a Phase II trial, tirzepatide showed promising results in reducing liver fat content and improving markers of liver injury in NASH patients. It is currently in Phase III trials.
Efruxifermin (EFX, Akero Therapeutics)
Efruxifermin is a fusion protein combining the human IgG1 Fc domain with a modified human FGF21 (Fc-FGF21), designed to balance in vitro agonist potency at FGFR1c, FGFR2c, and FGFR3c. Efruxifermin has a longer half-life of 3–3.5 days compared to most FGF21 analogs. Initially studied in patients with Type 2 Diabetes (T2D), it demonstrated improvements in lipoprotein profiles and glycemic control, which led to further investigation in NASH patients. The drug aims to mimic the metabolic benefits of FGF21, a key regulator of metabolism. In a Phase IIa trial, EFX showed significant improvements in liver fat content, liver enzymes, and markers of fibrosis in NASH patients. Currently, it is being evaluated in Phase IIb trials, focusing on the safety and efficacy of weekly subcutaneous administration for 16 weeks.
Conclusion
The NASH market is witnessing intense competition as numerous pharmaceutical companies strive to secure a significant market share. The anticipated launch of new and emerging drugs is expected to propel the market forward, addressing the substantial unmet needs in this area. Regulatory bodies also play a crucial role by offering special designations to promising therapies and encouraging their development. A comprehensive analysis of the strengths and weaknesses of these late-stage pipeline products and their potential impact on sales will be vital for ensuring their commercial success. Ultimately, the rising prevalence of NASH, increased disease awareness, and the promising pipeline of emerging therapies are poised to expand the market in the coming years significantly.

Downloads
Article in PDF
Recent Articles
- Notizia
- Orchard licenses gene therapy tech from GSK; Glympse raises $46M; FDA voted to recommend Terlipre...
- AZ announces positive results for durvalumab and tremelimumab; FDA nods to extend LILETTA’s use t...
- FDA’s No to Intercept’s NASH Drug; Roche’s Phesgo gets approval; Gilead’s Remdesivir pricing
- Zealand Pharma’s Phase III Results of Glepaglutide; FDA Approves Amylyx’s ALS Drug Relyvrio; Novo...