Understanding Neuroendocrine Tumors (NETs): An In-Depth Look
Nov 01, 2024
Table of Contents
Neuroendocrine tumors, commonly referred to as NETs, are a complex and often misunderstood type of cancer that can originate in various parts of the body. As with other cancers, these tumors are named based on their site of origin. For example, when NETs develop in the lungs, they are termed lung NETs, which are considered the primary form of the cancer. However, if the cancer cells travel and establish themselves in a new location in the body, it is classified as a secondary or metastatic cancer.
While NETs can theoretically arise from any part of the body, they most frequently start in the digestive system, affecting areas like the stomach, small bowel, large bowel, pancreas, and rectum. Comparatively, NETs originating in the lungs are less common. Beyond the gastrointestinal tract and lungs, NETs may also develop in the esophagus, appendix, skin, prostate, womb, and adrenal glands, among other areas.
Downloads
Article in PDF
Recent Articles
- Neuroendocrine Tumors (NETs): Unraveling the Mystery of a Complex Cancer
- Gastrointestinal Cancers: Exploring the Range of Digestive Tract Malignancies
- From Beta to Alpha Emitters: Radioligand Therapies Reshaping the Neuroendocrine Tumors Treatment ...
- Unveiling the Future: Global Neuroendocrine Tumor Market Trends & Innovations
Neuroendocrine tumors can be categorized in multiple ways. One primary method is by assessing whether they are functional or nonfunctional. Functional NETs produce excessive hormones that often result in symptoms related to hormone imbalances, whereas nonfunctional NETs do not produce significant hormone levels, often making them harder to detect early. NETs may also be classified based on their growth behavior, with some being indolent (slow-growing) and others displaying more aggressive tendencies.
The World Health Organization has provided additional classifications to help standardize the grouping of NETs, as understanding their nature is crucial for effective treatment. While the exact causes of NETs remain largely unknown, researchers have identified several associated risk factors, such as pituitary adenomas, Von Hippel-Lindau (VHL) syndrome, and pheochromocytomas, that may increase the likelihood of developing these tumors. Despite the severity associated with cancer diagnoses, NETs are known for their often slow progression, with some tumors remaining stagnant for months or even years, meaning immediate treatment might not always be necessary.
Curious to learn more? Read the full blog here and stay informed about the evolving landscape of NET awareness and treatment!
Diagnosing NETs: Tests and Methods for Accurate Detection
The diagnosis of NETs is multifaceted, often requiring a range of tests to accurately determine the presence, location, and type of tumor. The diagnostic journey typically begins with symptoms prompting healthcare providers to recommend a series of tests that can include urine tests, CT scans, ultrasounds, MRIs, PET-CT scans, and radioactive imaging. For NETs suspected within the digestive tract, tools like colonoscopy and endoscopy can help visualize the area and collect tissue samples, while more specialized approaches, such as biopsies and blood tests, provide detailed insights into the tumor’s cellular characteristics.
Imaging tests form a critical part of the diagnostic process. Computed tomography (CT) scans and Magnetic Resonance Imaging (MRI) are commonly employed to obtain high-resolution images of the tumor site, offering essential details about its size and spread. Advanced imaging techniques, such as Positron Emission Tomography (PET) and Somatostatin Receptor Scintigraphy (SRS), allow for more precise targeting, especially for NETs that express somatostatin receptors. Newer scans like Gallium 68 (Ga-68) and Copper-64 (Cu-64), along with Octreoscans, FDG (Fluorodeoxyglucose) scans, and MIBG (Meta-iodobenzylguanidine) scans, provide additional imaging options tailored for NET diagnosis.
Various tissue tests, including Endoscopic Ultrasound-Guided Fine-Needle Aspirations (EUS-FNA) and Fine-Needle Aspiration (FNA) biopsies, are often employed to retrieve tumor samples, which are subsequently analyzed using Immunohistochemistry (IHC) to determine specific protein expressions associated with NETs. Blood work, including Chromogranin A (CGA) and NETest, adds another layer of information, helping to establish the tumor’s profile and functional status. Given the complexities involved in diagnosing NETs, physicians rely on multiple test results to build a clear, comprehensive picture, enabling them to tailor treatment plans with accuracy.
Treatment Options for NETs
Treatment for NETs depends on a variety of factors, including the tumor’s location, size, and whether it has metastasized. Traditionally, surgery has been the cornerstone of NET treatment, particularly for patients with localized or early-stage tumors. However, over recent decades, advancements in medical technology have introduced several nonsurgical treatments, offering hope to patients with advanced or inoperable NETs. For low-grade NETs, surgery remains a viable option, even when there is spread to the liver or lymph nodes, as it can potentially cure or significantly reduce the tumor burden.
For patients with more advanced disease, a range of tumor debulking techniques can help alleviate symptoms and improve quality of life. Procedures like hepatic artery embolization (HAE), selective internal radiotherapy (SIRT), radiofrequency ablation (RFA), and palliative hepatic cytoreductive surgery are employed to shrink tumors and relieve symptoms caused by excess hormones. As many NETs express Somatostatin receptors (SSTRs), long-acting somatostatin analogs (SSAs) are commonly used to manage these tumors. SSAs, such as Octreotide and Lanreotide, not only help control hormone-related symptoms but also exhibit antiproliferative properties, effectively slowing the tumor’s progression.
Epidemiology of NETs
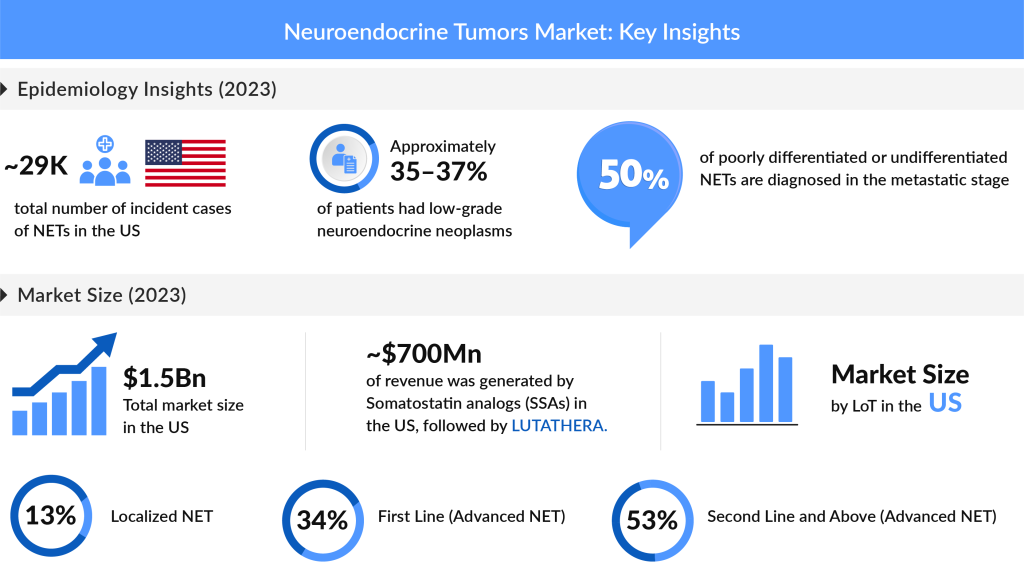
Epidemiological studies on NETs offer valuable insights into the prevalence, incidence, and characteristics of this complex disease. The latest data highlights that, in the United States alone, there were approximately 29K new cases of NETs diagnosed in 2023, with projections indicating a steady increase over the forecasted period. Among the subtypes, Non-functional/Asymptomatic/Unknown NETs accounted for the majority of cases, with an estimated 16K cases in 2023, while Functional NETs contributed nearly 15K cases. In Europe, a comparable trend has been observed, with the UK alone representing almost 30% of NET cases within EU4 (Germany, France, Italy, and Spain) and the UK in 2023.
The increasing number of comprehensive cancer registries and improved reporting systems have significantly enhanced the accuracy of NETs incidence data, offering a more precise understanding of these tumors. While low-grade neuroendocrine neoplasms account for around 35–37% of cases, intermediate-grade neoplasms contribute to 17–24%, and high-grade neoplasms make up 6–7%. Despite ongoing efforts to improve NET data, the grade status remains unknown for many patients, highlighting the need for continued advancements in NET diagnostics and reporting.
NET Drug Landscape: Marketed and Emerging Therapeutics

The treatment landscape for NETs has evolved significantly, with both marketed and emerging drugs making substantial impacts on patient outcomes. Key marketed drugs include WELIREG (belzutifan/MK-6482), LUTATHERA (lutetium Lu 177 dotatate), SUTENT (sunitinib malate), AFINITOR (everolimus), SOMATULINE DEPOT (lanreotide), DEMSER (metyrosine), and AZEDRA (iobenguane I 131). These drugs provide multiple mechanisms to target NETs, from inhibiting tumor growth to reducing hormone levels and symptoms.
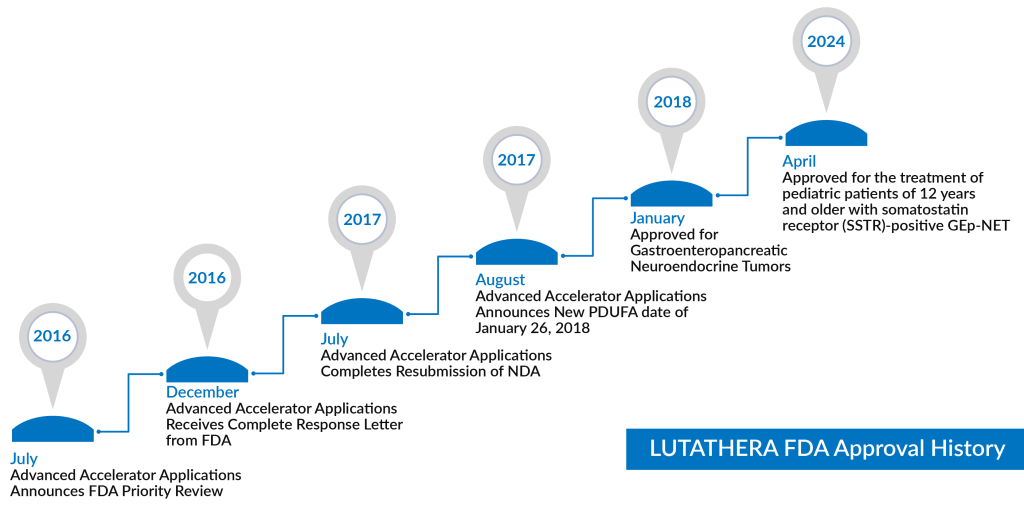
One notable drug, LUTATHERA (lutetium Lu 177 dotatate), is a 177Lu-labeled somatostatin analog peptide that has garnered attention as a leading therapy in Peptide Receptor Radionuclide Therapy (PRRT). FDA-approved in 2018 for SSTR+ GEP-NETs, it demonstrated positive results in the NETTER-1 trial and recently gained expanded approval in 2024 to include pediatric patients over age 12. Another prominent drug, SOMATULINE DEPOT (lanreotide), was initially approved in 2014 for unresectable, advanced GEP-NETs and has since expanded its indications to treat carcinoid syndrome.
Discover LUTATHERA’s Journey in Treating Neuroendocrine Tumors (NETs) in Our Latest Blog!
Emerging drugs in the NETs space show promise as well, such as ITM-11 (177Lu-edotreotide), which is currently being evaluated in Phase III trials (COMPETE and COMPOSE) for GEP-NETs and displays favorable efficacy and safety. Additionally, CAM2029, a long-acting octreotide depot developed by Camurus, is advancing through trials and expected to present topline results in 2025, with potential for broader approval soon after.
The Market Outlook for NET Treatments: Trends and Insights
For many NET patients, Somatostatin analogs remain a cornerstone of therapy. Alongside these traditional treatments, innovations in Peptide Receptor Radionuclide Therapy (PRRT), targeted radiotherapy, and drug development have transformed NET management. As imaging technology advances, enabling more precise PET/CT targeting, the industry has seen a rise in demand for targeted NET therapies, driven by their effectiveness and manageable side effect profiles.
The total market size for Neuroendocrine Tumors (NETs) in the U.S. was estimated at nearly USD 1,530 million in 2023, with expectations for significant growth at a notable CAGR during the forecast period. Among the EU4 countries and the UK, the highest market share for NETs was recorded in the UK, which accounted for approximately 31% of the total market share in EU4 and the UK in 2023. In the same year, Somatostatin analogs (SSAs) dominated the market, capturing about USD 700 million in the U.S., followed closely by LUTATHERA. This growth is largely attributed to improvements in early detection, the rising prevalence of NETs, and increased investment in R&D.
Living with Neuroendocrine Tumors: Patient Perspectives and Support
Living with Neuroendocrine Tumors (NETs) can be a complex journey, marked by both physical and emotional challenges. For many patients, the diagnosis may initially be overwhelming, given the rarity and intricacies of NETs. However, patient support networks and educational resources play a crucial role in navigating this journey. Various organizations, such as the Neuroendocrine Tumor Research Foundation (NETRF) and the Carcinoid Cancer Foundation, provide vital information about the disease, treatment options, and personal stories from fellow patients. These communities foster connections among individuals facing similar challenges, allowing for the sharing of experiences, advice, and coping strategies.
Moreover, psychosocial support is an essential aspect of holistic care for NET patients. The psychological impact of living with a chronic illness can manifest in anxiety, depression, or feelings of isolation. Healthcare providers are increasingly recognizing the need for comprehensive support systems, including access to counselors, social workers, and support groups, to address the emotional and mental health needs of patients. Engaging in mindfulness practices, such as meditation or yoga, can also provide significant benefits, promoting mental well-being and helping to manage stress.
Nutrition is another important component of living with NETs. Some patients may experience symptoms such as diarrhea or hormonal imbalances, making it crucial to adopt a balanced diet tailored to their individual needs. Consulting with a nutritionist familiar with NETs can help patients create meal plans that support their treatment and overall health. Understanding the importance of regular medical follow-ups is essential, as routine monitoring can help catch any changes early and ensure that treatment remains effective.
The Future of NET Research: Innovations on the Horizon
As the understanding of Neuroendocrine Tumors (NETs) continues to evolve, research is paving the way for more innovative treatments and improved patient outcomes. Ongoing clinical trials are crucial in this endeavor, investigating new drug combinations, targeted therapies, and novel treatment modalities. For example, combination therapies that pair traditional treatments like SSAs with newer agents such as immune checkpoint inhibitors are being explored. These approaches aim to enhance the immune system’s ability to fight cancer and have shown promising early results.
One exciting area of research involves precision medicine, which seeks to tailor treatment based on individual tumor characteristics. By analyzing specific genetic mutations and biomarkers associated with NETs, researchers hope to develop personalized treatment plans that optimize efficacy and minimize side effects. The use of liquid biopsies to monitor tumor progression and treatment response is another area gaining traction, potentially allowing for non-invasive, real-time assessments of disease status.
Furthermore, the development of immunotherapies is making significant strides in the fight against NETs. Treatments that harness the body’s immune system to target cancer cells are gaining attention, with several trials underway to evaluate their effectiveness. Advances in CAR-T cell therapy and monoclonal antibodies are promising avenues for enhancing treatment strategies.
International collaborations and initiatives, such as the European Neuroendocrine Tumor Society (ENETS) and the North American Neuroendocrine Tumor Society (NANETS), are also vital for promoting research and education. These organizations facilitate global information exchange, focusing on raising awareness, improving diagnostic methods, and developing new therapies.
The Journey Ahead in NET Awareness and Treatment
In conclusion, the landscape of Neuroendocrine Tumors is evolving rapidly, driven by advancements in research, improved diagnostic techniques, and a growing understanding of the disease. While the challenges associated with NETs remain significant, the ongoing development of new therapies and support systems offers hope for patients and their families. The importance of awareness cannot be overstated; with increased knowledge about NETs, healthcare providers can better recognize symptoms and implement early interventions, ultimately improving patient outcomes.

As we look toward the future, it is essential to foster collaboration among researchers, clinicians, and patient advocacy groups to ensure that innovations translate into meaningful progress. Empowering patients with education and support resources, while continually advancing research, will be pivotal in enhancing the lives of those affected by NETs. Together, we can build a more informed and supportive community that champions the fight against Neuroendocrine Tumors, offering hope and resilience to all those on this journey.

Downloads
Article in PDF
Recent Articles
- Unveiling the Future: Global Neuroendocrine Tumor Market Trends & Innovations
- From Beta to Alpha Emitters: Radioligand Therapies Reshaping the Neuroendocrine Tumors Treatment ...
- Neuroendocrine Tumors (NETs): Unraveling the Mystery of a Complex Cancer
- Gastrointestinal Cancers: Exploring the Range of Digestive Tract Malignancies