Neuroendocrine Tumors (NETs): Unraveling the Mystery of a Complex Cancer
Oct 30, 2024
Neuroendocrine tumors are a complex group of tumors that develop predominantly in the digestive or respiratory tract but can occur in many areas of the body. These tumors arise from cells called neuroendocrine cells. Like all cancers, NETs develop when the specialized cells undergo changes, causing them to divide uncontrollably and grow into an abnormal tissue mass (tumor).
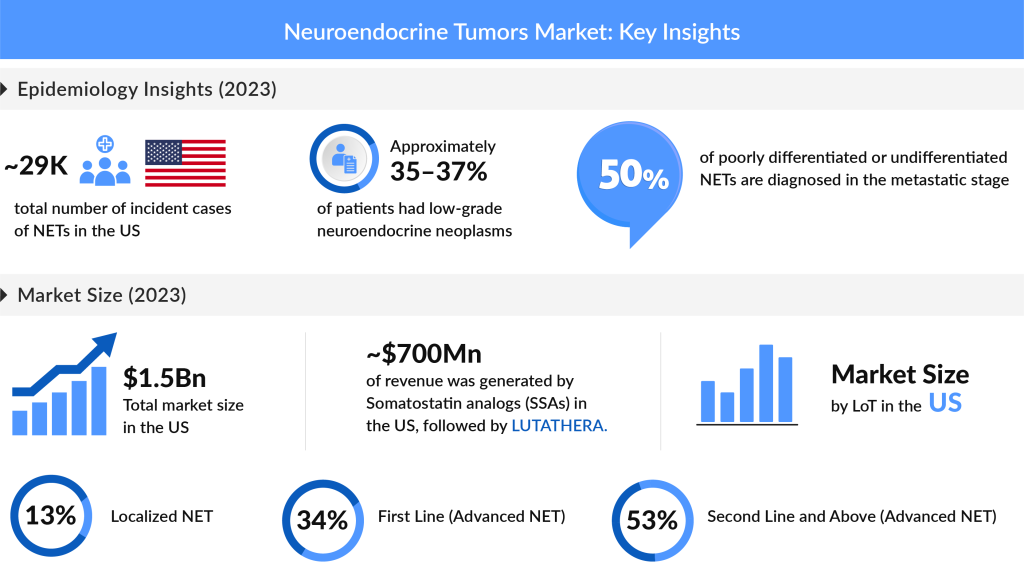
The epidemiology of neuroendocrine tumors paints a compelling picture of a growing health concern, particularly in the United States and the EU4 (Germany, France, Italy, and Spain) plus the United Kingdom. In 2023, the U.S. recorded nearly 29,500 new incident cases, with projections indicating a steady increase through 2034. The landscape of these tumors is dominated by non-functional, asymptomatic, or unknown cases, accounting for approximately 16,000 incidents, while functional NETs contributed around 15,000 cases. This evolving data not only sheds light on the epidemiology of NETs but also emphasizes the urgent need for continued research and improved diagnostic efforts in this critical area of oncology.
The treatment landscape for neuroendocrine tumors (NETs) has evolved significantly over the years, shifting from a primarily surgical approach to a broader range of neuroendocrine tumor treatments that include both surgical and nonsurgical options. Traditionally, surgery was the cornerstone of therapy for neuroendocrine tumors, particularly for those classified as G1 or G2, where curative surgery can still be considered even in the presence of liver or lymph node metastases. However, as advancements in medical science emerged, the development of various neuroendocrine tumors therapies provided new avenues for patient care, especially for those with advanced disease. Tumor debulking techniques such as hepatic artery embolization (HAE), selective internal radiotherapy (SIRT), radiofrequency ablation (RFA), and palliative hepatic cytoreductive surgery have been utilized to alleviate symptoms and reduce tumor burden, particularly in patients with metastatic disease.
Downloads
Article in PDF
Recent Articles
- With the Advances in Regenerative Therapy to Offer Curated Treatment Options, Off-label Therapy M...
- Pfizer/ OPKO Health’s Somatrogon; FDA EU for SCONE device; Antengene ATG-010 for rrMM and rrDLBCL...
- Biogen triumphs; Lilly, Pfizer, Ipsen back; Court nixes; Mylan EpiPen recall
- Takeda acquisition on Shire raises its share by 25 percent
- Prosecutors rope Pfizer; Pharma groups to FDA; Regeneron simulates; Otsuka & Lundbeck revive...
In the realm of neuroendocrine tumors drugs, several agents have gained FDA approval, broadening the options available for patients. Among these, LUTATHERA (lutetium Lu 177 dotatate) emerged as a groundbreaking treatment in January 2018. This radiolabeled somatostatin analog peptide is part of Peptide Receptor Radionuclide Therapy (PRRT), specifically designed to target tumors expressing somatostatin receptors. Clinical data from the NETTER-1 trial (NCT01578239) demonstrated its efficacy in treating somatostatin receptor-positive gastrointestinal NETs, leading to its approval for adults. The versatility of LUTATHERA was further validated in April 2024 when it received FDA approval for treating pediatric patients aged 12 and older with similar indications.

Another pivotal player in the neuroendocrine tumors treatment arsenal is SOMATULINE DEPOT (lanreotide), which received FDA approval in December 2014 for patients with unresectable, well or moderately differentiated, locally advanced, or metastatic GEP-NETs, enhancing progression-free survival rates. This somatostatin analog also helps manage symptoms associated with carcinoid syndrome, with further approvals expanding its indications over the years. Notably, in May 2024, Cipla announced its final approval for an abbreviated new drug application (ANDA) for lanreotide injection, introducing additional options for patients in need of these essential neuroendocrine tumors therapies.
As we look forward, the increasing understanding of NETs, alongside ongoing research and the introduction of innovative neuroendocrine tumors drugs, promises to further enhance the treatment landscape, offering hope to patients facing these complex malignancies. With a diverse portfolio of therapies now available, including novel surgical techniques and targeted drug treatments, the fight against neuroendocrine tumors continues to advance, aiming for improved outcomes and quality of life for affected individuals.
The Neuroendocrine Tumors (NETs) market is poised for significant transformation in the coming years, particularly with the anticipated launch of several promising therapies such as Azedra (iobenguane I 131) from Progenics Pharmaceuticals, Sulfatinib by Hutchison MediPharma, Axitinib from Pfizer, RRx-001 developed by EpicentRx, Entrectinib from Ignyta, AMG-479 by Amgen, Carfilzomib (also from Amgen), ATG-008 from Antengene, and Fosbretabulin by Mateon Therapeutics. Azedra, currently in the pre-registration stage for the treatment of malignant, recurrent, and unresectable pheochromocytoma and paraganglioma—rare neuroendocrine tumors—has shown consistent performance since its FDA approval in 2019, raising hopes among patients and investors alike.
As of 2023, the total market size for NETs in the United States was estimated at approximately USD 1.5 billion and is projected to grow significantly with the introduction of these emerging therapies. Among the EU4 and the UK, the UK held the largest market share for NETs, accounting for nearly 31% of the overall market in this region. In the same year, somatostatin analogs (SSAs) dominated the market with a size of around USD 700 million in the US, followed closely by LUTATHERA. To capture substantial market shares, radiopharmaceutical key players must ensure robust product capabilities, supply chains, and production capacities while adapting to the need for innovative therapeutic approaches for cancer patients with limited options.
Oncolytic virotherapy is an emerging area of interest, with therapies like ELC-100 (Elicera Therapeutics) and SVV-001 (Seneca Therapeutics) currently under investigation for the treatment of NETs. However, it remains too early to predict their efficacy and safety due to insufficient clinical trial evidence.
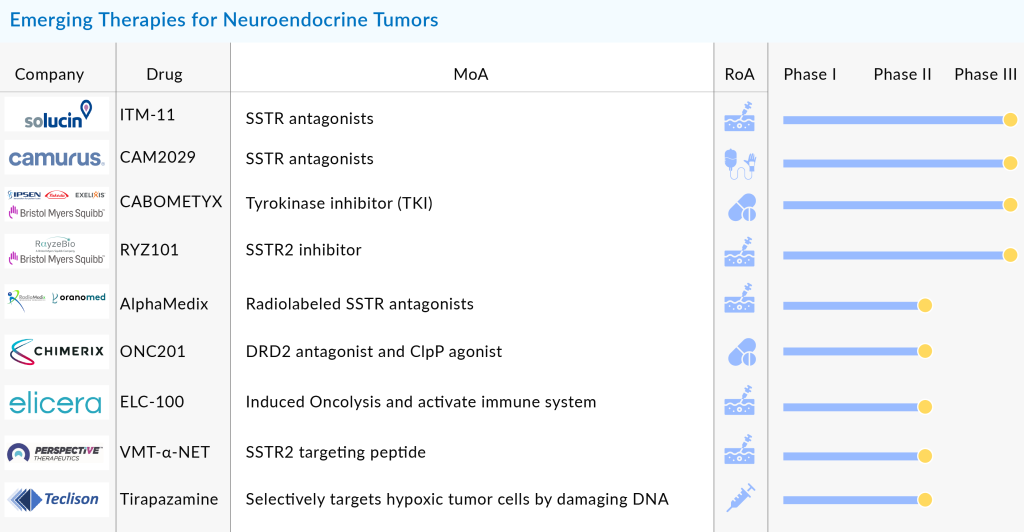
Several other promising therapies are on the horizon for Neuroendocrine Tumors (NETs), including Azedra (iobenguane I 131) from Progenics Pharmaceuticals, Sulfatinib by Hutchison MediPharma, Axitinib from Pfizer, RRx-001 developed by EpicentRx, Entrectinib from Ignyta, AMG-479 and Carfilzomib from Amgen, ATG-008 by Antengene, and Fosbretabulin from Mateon Therapeutics. Additionally, ITM-11 is under development by ITM Solucin GmbH, while CAM2029 is being developed by Camurus. The combination therapy CABOMETYX involves multiple players, including Ipsen, Takeda, Exelixis, and Bristol Myers Squibb. RYZ101 is a collaborative effort between Bristol Myers Squibb and RayzeBio. Other key players include AlphaMedix from Radiomedix and Orano Med, ONC201 from Chimerix, ELC-100 from Elicera Therapeutics, VMT-α-NET from Perspective Therapeutics, and Tirapazamine by Teclison.
For many NET patients, SSAs represent the standard of care, providing not only an antisecretory effect but also antiproliferative properties. Numerous studies have demonstrated that treatment with SSAs leads to improved time to tumor progression and overall survival compared to placebo therapies. Newer options, such as the mTOR antagonist everolimus, have also emerged. It’s important to note that cytotoxic chemotherapy has proven ineffective for gastrointestinal NETs. Recently, Peptide Receptor Radionuclide Therapy (PRRT) has gained traction as a novel option for NET patients who have not responded to other treatments. Despite these advancements, the prognosis for metastatic disease remains challenging.
The NCCN and ENETS guidelines recommend SSAs as first-line therapy when somatostatin receptor-related functional imaging is positive, particularly in cases of slow tumor progression and low tumor grade. For tumors with higher grades, faster progression, or inconclusive SSTR imaging, everolimus may be considered as a first-line option; otherwise, it typically serves as a second-line therapy. In patients with lung NETs who experience progression after treatment with an SSA and everolimus, treatment options include various chemotherapy regimens, PRRT, locoregional treatments (mainly nonsurgical), or participation in clinical trials for experimental therapies.
In conclusion, the landscape of Neuroendocrine Tumors (NETs) is rapidly evolving, driven by a deeper understanding of these complex malignancies and a surge of innovative treatments entering the market. As we stand on the brink of a new era in oncology, the introduction of groundbreaking therapies such as LUTATHERA and SOMATULINE DEPOT is set to transform patient care, providing renewed hope for individuals facing these often elusive tumors. With an anticipated market growth that reflects both the increasing incidence of NETs and the relentless pursuit of effective treatments, companies like Progenics Pharmaceuticals, Amgen, and Hutchison MediPharma are leading the charge in advancing therapeutic options.
Moreover, the promise of emerging treatments, including radiopharmaceuticals and oncolytic virotherapy, hints at a future where NETs may be managed with greater precision and effectiveness. As clinical trials continue to unfold and new agents prove their worth, the focus on personalized medicine will further enhance the patient experience, allowing for tailored approaches that maximize outcomes while minimizing side effects. The commitment to research and innovation in this field is paramount, as we strive not only for better survival rates but also for improved quality of life for NET patients.
As we look ahead, the collaboration between researchers, clinicians, and pharmaceutical companies is essential to unlocking the full potential of these therapies. Together, we can redefine the narrative around neuroendocrine tumors, moving towards a future where these complex conditions are met with a comprehensive arsenal of effective treatments. The journey continues, and with every new discovery, we draw closer to offering patients not just hope, but tangible solutions in the fight against NETs.

Downloads
Article in PDF
Recent Articles
- Snippet
- Novartis eyes; Pfizer considers cashing; Mylan pushes to seal $465M; Valeant with Takeda for deal
- FDA Grants Priority Review to Merck’s Application for KEYTRUDA Plus Padcev; Roche and Carmot Ther...
- Respiratory Syncytial Virus Treatment Market: A Complex Space Worth Billions!
- EMA to relocate to Amsterdam; Roche’s prospects; Amgen’s Humira Biosimilar delayed; Purdue’s opio...