Neuromodulation Devices Observed to Reshape the Migraine Treatment Dynamics
Nov 29, 2021
Table of Contents
Migraine is the most commonly encountered disabling disorder that can be featured as attacks of severe, unilateral, and a pulsating form of headache composed of symptoms such as photophobia, phonophobia, nausea, vomiting, and cutaneous allodynia. As per the Migraine Research Foundation 2021, Migraine is considered to be the third most preventable illness globally. Furthermore, as per the former source, more than four million adults are suffering from chronic daily Migraine in the year 2021. Also, as per DelveInsight’s analysis, the total number of Migraine prevalent population in the year 2021 accounts for 45,503, and this number is expected to increase at an alarming rate reaching up to 48,448 by 2030. There is not much research evidence pertaining to the cause of Migraine but the prominent triggers include bright lightning, dehydration, severe heat amongst others.
Migraine risk factors include genetics, gender (more common in women in contrast to men), higher stress levels and smoking. Migraine diagnosis is usually assessed physically by examining the medical and family history of the patient. Migraine treatment usually comprises of drugs that tend to stop the symptoms temporarily and help in preventing future attacks. Pain alleviating medications include pain relievers (Advil, Motrin IB), triptans (Maxalt, Maxalt-MLT) amongst others. Migraine preventive medications consist of blood-pressure alleviating medications and antidepressants.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- FDA Grants 510(k) Clearance to Neonav® ECG Tip Location System; ClearPoint Neuro Secures EU MDR C...
- Johnson & Johnson’s Tecnis PureSee lens; Sparrow BioAcoustics’s Stethoscope Software; Better ...
- Laborie’s Enhanced Gastrointestinal Diagnostics; Carestream’s Image Suite MR 10 Software Launch; ...
- Viz.ai’s AI Algorithm for Abdominal Aortic Aneurysm; BrainTale Unveils the New Version of I...
- Amber Implants Wins FDA 510(k) Clearance for VCFix® Spinal System; Merit Medical’s Embosphere Mic...
Another form of treatment includes Migraine Neuromodulation Devices that offer acute and preventive treatment, as approved by the FDA and CE Mark. Migraine devices are related to the hyperactivity of the brain. Although every device tends to work differently, however, each one offers neuromodulation. Neuromodulation Devices use electric currents and magnetic fields for stimulating the nervous system, causing various effects on brain activity. Due to the increasing prevalence of Migraine patients globally, treatment-resistant Migraine, and the rising preference for minimally-invasive and non-invasive device treatment in the market, there is a heavy demand for Migraine devices in the market, leading to Migraine Device Market Growth.
Migraine Prevalence – An Overview
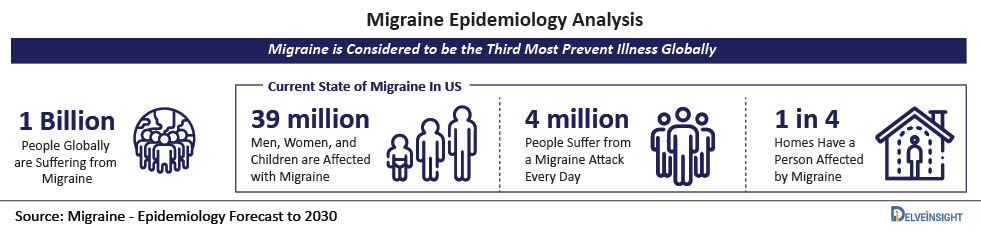
- Also, as per DelveInsight’s analysis, the total number of Migraine prevalent population in the year 2021 accounts for 45,503, and this number is expected to increase at an alarming rate reaching up to 48,448 by 2030.
- These were well supported by research studies by various organizations such as the Centers for Disease Control and Prevention 2018, reported women were nearly twice as likely as men to suffer from Migraine or headaches. The percentage of people in the United States suffering from Migraine in the age group of 18–44 years was 25.5%.
- According to the Migraine Research Foundation, 2021, Migraine tends to affect 39 million men, women, and children in the United States and one billion people globally. As per the aforementioned source, Migraine is the third most prevalent disease in the world.
- As per the statistics given by Head Pain Institute, in 2021, about 39 million adults in the United States tend to suffer from Migraine. As per the same source, more than 4 million of these people suffer from a Migraine attack every day.
- As per the source Migraine Statistics 2021, approximately 39 million people in the United States tend to suffer from Migraine. As per the former source, about 1 billion people worldwide are suffering from Migraine.
Migraine Treatment Options
Migraine treatment options are focused upon treatment of the ongoing symptoms and preventing any future Migraine attacks.
Pharmacological
Pharmacological treatment options comprise pain relievers, that are acute and abortive form of drugs taken during an ongoing Migraine attack and have been designed to cause inhibition of the symptoms. Certain drugs used to relieve pain associated symptoms in Migraine include Dihydroergotamine, Lasmiditan and Ubrogepant along with others. Preventive treatments are usually recommended when the patient suffers from frequent, long-lasting and severe forms of headache that do not respond effectively to the pain-relieving medications. These comprise anti-seizure drugs, CGRP monoclonal antibodies amongst others.
Alternative Therapies in Migraine
Acupuncture
Various researches have supported the utilization of acupuncture in headache pain. Acupuncture treatment involves a practitioner inserting thin, disposable needles at several areas of the head (within well-defined points).
Biofeedback
Biofeedback is found to be effective in relieving Migraine-related pain. The relaxation technique tends to use a special instrument for monitoring and assessing physical responses associated with stress, for instance, muscle tension.
Cognitive Behavioral Therapy
The therapy is useful in certain patients. It involves controlling the behaviour and thoughts of the patients, thereby affecting the patient’s ability to perceive pain.
Migraine Devices
Overview and Evolution
Another form of treatment option offered for Migraine patients includes Migraine Medical Devices. Migraine Medical Devices are devices that help to prevent or relieve the pain after affecting the neuronal activities of the brain. These devices are also known as Neuromodulation Devices. Neuromodulation is referred to as the modulation or alteration of the central nervous system via invasive/non-invasive magnetic or electrical pulses. Although neuromodulation is not typically considered as the first-line treatment for Migraine disorders yet, however many forms of non-invasive and minimally invasive options have been approved for Migraine treatment via FDA and CE Mark.
The use of Neuromodulation Devices in the treatment of Migraines has been researched over the past 17 years and was extensively documented to validate the therapeutic efficacy of implantable forms of Neuromodulation Devices in Migraine. The first-ever report on this subject of Neuromodulation was Dr. Reed’s paper published in the year 1999 on Occipital Nerve Stimulation. After that, Cefaly was the first-ever non-invasive neurostimulator device that got approval for the treatment of Migraine. In the year 2014, Cefaly got FDA approved for the treatment of Migraine prophylaxis that was renamed Cefaly PREVENT in the later years.
Types of Migraine Devices
Neuromodulation Devices in Migraine treatment offer alteration in the neuronal circuits via electromagnetic current that is delivered noninvasively or via implantable devices. They can be effectively used in patients that deal with certain health issues, other conditions that prevent them from opting for medications, tolerability issues associated with medications, or patients suffering from medication overuse headaches.
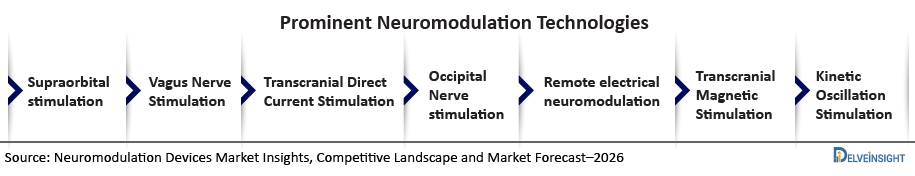
Currently, there are seven types of Migraine treatment technology that are commercially approved and available in both the United States and European countries. Currently, there is no form of neuromodulation treatment that is available in Japan, however, the technologies of Migraine treatment will be launched very soon in near future in Japan.
Various technologies that are used in Migraine treatment comprise supraorbital stimulation, Vagus Nerve Stimulation, Transcranial Direct Current Stimulation, Occipital Nerve stimulation, Remote electrical neuromodulation, Transcranial Magnetic Stimulation, and Kinetic Oscillation Stimulation.
| Neuromodulation Technology | Targeted Area | Device Examples |
|---|---|---|
| Supraorbital Stimulation | An upper branch of the trigeminal, or supraorbital, nerves | Cefaly |
| Vagus Nerve Stimulation | Vagus nerve | GammaCore Sapphire |
| Occipital Nerve Stimulation | Occipital nerve | The device by Salvia Bioelectronics(in development) |
| Remote Electrical Neuromodulation | Peripheral nerves in the upper arm | Nerivio |
| Transcranial Magnetic Stimulation | Layers of the scalp, the skull, the meninges (membranes that enclose the brain and spinal cord), the cerebrospinal fluid, and the superficial layers of the cortex | sTMS mini, Cerena |
| Transcranial Direct Current Stimulation | Dorsolateral prefrontal cortex or the occipital nerve | PainX™ tDCS, HeadaTerm |
| Kinetic Oscillation Stimulation | Sensory nerve endings in mucosa | KOS Device |

The key players operating in the segment of Migraine Neuromodulation Devices comprise Theranica Bio-Electronics LTD, electroCore, Inc., Cirrus Healthcare Products, Cefaly Technology, eNeura Inc., Prolivio, Neuros Medical, Neurolief Ltd., tVNS Technologies GmbH, Medtronic, WAT Medical, Chordate Medical, Chordate Medical, Soterix mEDICAL among others.
Key Migraine Devices
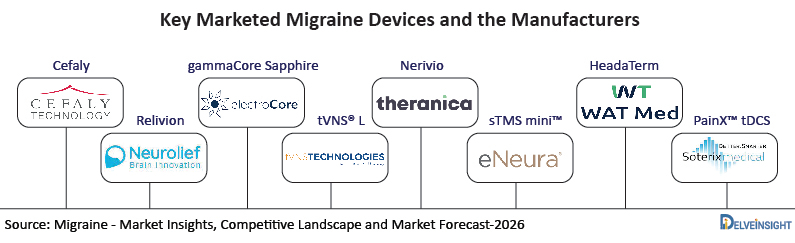
Cefaly
The Cefaly external e-TNS device was the first approved in the year 2014 considering promising research data for the prevention of Migraine attacks in the year 2014. It was further approved for use in the treatment of Migraine attacks in the year 2017. The device has been designed to be worn on the forehead, along with a reusable electrode that is in contact with the skin. The device is intended to be used twice daily for 20 minutes for the prevention of Migraine attacks. It is also allowed to be used for 60 minutes during a Migraine attack to reduce symptoms.
The Cefaly dual kit comprises of the device, an electrode (can be used about 20 times), a power adapter, a charging cable, and a storage case.
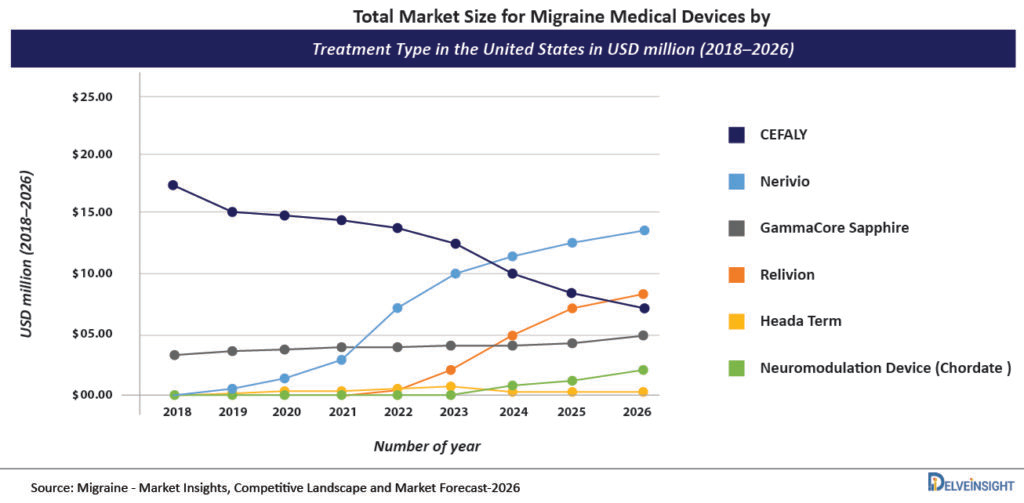
As per DelveInsight’s analysis, the Cefaly device holds the highest market share in the United States in comparison to other devices and was valued at approximately USD 15.03 million during the year 2020.
Relivion
Relivion is an e-TNS, non-invasive device that delivers electric impulses of electric current for stimulation of occipital and trigeminal nerves. The headset comprises two sensors that sit on either side of the nose (similar to the nosepieces of eyeglasses) and is allowed to be worn and activated during the onset of a Migraine attack. The amount and type of modulation can be controlled via the app while the device is paired to the smartphone. In a research study, it was discovered that about 76 device users had received headache relief during the onset of Migraine attack after only one time of treatment, acquainted with no adverse effects.
The device was earlier approved in Europe and in the year 2021, it received FDA clearance in the United States for at-home treatment of acute Migraine conditions in adults.
gammaCore Sapphire
The device was FDA-cleared in the year 2015. It is a handheld device that was intended for users to self-administer small doses of noninvasive vagus nerve stimulation therapy for the treatment of cluster headaches and Migraines. The rechargeable and reloadable device was intended for multiyear use in patients. To activate the device, the doctor will provide a prescription code that gets delivered via a prescription radio-frequency identification card. The users are then supposed to hold the device against their neck area, just below the jawline so that smaller doses of electric impulses can be transferred to the vagus nerve. In July 2020, the FDA gave the GammaCore emergency use authorization (EUA), for people that were suffering from COVID-19 and experiencing asthma-like breathing difficulties.
In February 2021, the FDA authority had expanded the clearance of the device for acute and preventive treatment of Migraine in adolescents that were aged between 12 and 17 years. The device was previously approved for the indication in adults only.
tVNS® L
The device is a neurostimulation device for the relief of Migraine symptoms while maintaining the sympathovagal imbalance. The device includes tVNS® L Stimulation Unit, Ear Electrode with Cable Clip, Carrying Case, Charger, Electrode Pads, Electrode Contact Cream, Travel Container for Electrode Gel, Access to the tVNS® App, among others. The device allows activation of special nerve fibre via electrical impulses by applying the ear electrode. The user can control the intensity of impulses by themselves. The device was approved for the treatment of Migraines. The device is easy to use, smaller similar to a smartphone and can be easily used regularly by patients. The device acts as an additional therapy to the already existing medication and does not require any surgery. The device functions without causing any adverse effects on the patients. The tVNS patient app allows tracking the treatment and data with the concerned doctors.
Nerivio
The Nerivio is an FDA-approved wireless remote neuromodulation armband device for the acute treatment of Migraine with or without aura in people aged 12 years and older. The device is controlled by an app that has been designed for providing personalized treatments. It is a non-invasive, drug-free, easy to use, and non-rechargeable battery that is made to be worn onto the upper arm and operates via a mobile application. The device also comprises an electronic case, firmware, mobile application software, and an armband that is well-equipped with electrodes dipped in the hydrogel.
The Nerivio unit is applicable to be used for 12 treatments, after which the device can be discarded off and a new device can be given for refilling the prescription.
sTMS mini™
The eNeura sTMS mini was an FDA-approved device that was previously sold under the brand name Spring TMS. The device is a battery-powered, handheld device that can be used for both prevention and treatment of Migraine attacks. The user intends to place and hold the device against the back of the head to cradle the base of the skull area. After the push of the button, the device is specially shaped into electrical coils for delivering magnetic stimulations that have been designed for the treatment and prevention of Migraine attacks while interrupting the abnormal activities of the brain associated with Migraine.
HeadaTerm
The Headaterm device utilizes neuromodulation technology thereby sending the precise form of electric impulses to target areas in the brain region and interfering with the vomiting signals from the brain to the stomach region. The headaterm band is a safety device that is drug-free and can be worn on the forehead region to treat and relieve primary headaches including Migraine, tension-related headaches, chronic daily headache, and cluster headache. The device is attached to the forehead via a self-adhesive electrode. A tingling sensation is created due to the tiny electrical impulses from the Headaterm device that stimulate the transgeminal nerve. The device tends to provide relief from an active Migraine attack in 20-30 minutes. The device is energy efficient and can withstand long standby mode. It can also operate for seven hours. The device is CE-certified and can also be used while traveling outdoors.
PainX™ tDCS
It is a type of Neuromodulation Device that is non-invasive and tends to stimulate the brain via electrodes on the hair. It is a safe, and effective in-office treatment for Migraines. The device offers a 37% of reduction in pain due to fewer or shorter attacks. The device does not require the patient to undergo any surgery. It is a battery-powered device that delivers mild electric impulses to the cerebral cortex region for modulating reactions towards painful experiences or for controlling pain perception in the patient. It is a highly promising therapy that is associated with oral pain medications without any possible side effects. Additionally, the device proves to be a valuable option for patients that become unresponsive to the traditional treatments.
Key Developments in Migraine Treatment Scenario
There have been various launches, approvals of Migraine-associated devices and mergers, acquisitions between the key players in recent years. Owing to the increase in approvals and acquisitions associated with Migraine Medical Devices and companies, there is a surge in the demand for such Neuromodulation Devices for the effective treatment of Migraine. For instance, on March 02, 2021, Food and Drug Administration (FDA) had approved the Relivion system, from Neurolief for the treatment of acute Migraine pain. Due to the approval of the Relivion system in the United States, there will be an increase in the demand for such devices for Migraine treatment in the United States leading to an overall increase in Migraine Neuromodulation Devices. Furthermore, On January 25, 2021, FDA had approved the expanded indication for remote electrical neuromodulation (REN) Nerivio device. The device has been cleared for the treatment of patients suffering from episodic and chronic Migraine aged 12 years and above. Due to the approval of the Relivion system in the United States, there will be an increase in the demand for such devices for the treatment of Migraine in the United States leading to an overall increase in Migraine Neuromodulation Devices.
Moreover, On October 13, 2020, FDA had granted CEFALY DUAL medical device as an over-the-counter device for the treatment of acute and preventive treatment of patients suffering from Migraine. Due to the increased availability of the device without any prescription costs, patients will be more likely to prefer the CEFALY DUAL device leading to an overall increase in Migraine Neuromodulation Devices. Also, on June 27, 2019, DW Healthcare Partners had acquired Cefaly Technology. Cefaly technology was a Belgium-based neurostimulation device that was intended for the treatment of Migraine. Owing to the result of the acquisition, there will be an increased product portfolio for Migraine Devices for the company DW Healthcare Partners, increasing the demand and sales of such devices, leading to an overall increase in Migraine Neuromodulation Devices.
Future Prospective in the Arena of Migraine Neuromodulation Devices
Migraine is a neurological condition that has several manifestations including nausea, vomiting, and sensitivity to light. The most effective form of treatment (also known as the first-line of treatment) involves medications, however, it is not wholly sufficient and in most cases is found to be unresponsive for drug-refractory Migraine patients. Therefore, neuromodulation lay an important role in patients that are not responsive to the conventional form of treatments for Migraine or can sometimes be prescribed along with the ongoing medications. Certain pharmaceutical medications also comprise unwanted side effects. The medical devices help in the prevention or treatment of Migraines via manipulating the neuronal activities in the brain region.
There have been various technological advancements that have taken place for making Neuromodulation Devices easier and effective in use by Migraine patients. The devices have been manufactured in such a way that they are wearable and resemble headphones, thereby modulating the vestibular nerves. Other devices are manufactured in the form of wearable hand-cuffs that can be easily worn on the arm region, unlike the head or neck as certain patients grow extremely hypersensitive and avoid the use of Neuromodulation Devices on the head region during a severe Migraine attack. The new forms of Neuromodulation Devices used in the treatment of Migraines are patient-controlled, as they can modify the frequency of electric impulses that are sent to the targeted area. The devices are completely non-invasive and are drug-free. Most of the devices can be operated via mobile applications and thus record an adequate amount of data that can be easily checked by the doctor. Most electronic devices are powered by a non-rechargeable battery.
Several key market layers are continuously trying to develop technologies to offer patients with adequate Neuromodulation Devices that are effective in the management and treatment of Migraines. There are several pieces of research and clinical trials for devices indicated for Migraine which are currently in pipeline and will be soon available in the market. Salvia Bioelectronic’s Neuromodulation Device is in pipeline stages and is based on occipital nerve stimulation. The FDA had granted the breakthrough device designation to the company in the year 2020 for this Neuromodulation Device. Furthermore, the Neuromodulation Device from Shiratronics Inc. and the Pulsante® SPG Microstimulator System manufactured by Unity HA has also received FDA breakthrough device designation and will be very soon available in the market. Therefore, looking at the technological advancements taking place in this area it can be said that Neuromodulation Devices will surely prove to be providing an effective solution for patients suffering from Migraines shortly.
Conclusion
Migraine is a neurological condition that is not only common among the population but is also characterized by a frequent and intense form of headaches. The conventional treatment of Migraine is focused on alleviating the symptoms and preventing any future complications due to the condition. Several medications have also been designed for the treatment of Migraine, however, they are not that effective. Despite the pharma therapeutic options that are already available for treatment, certain patients develop resistance to drugs during the treatment or have a headache as a result of drug overuse. In such a scenario, patients have to switch to other forms of Neuromodulation Devices that provide effective relief against Migraine. The devices can be readily used for the prevention and treatment of both acute and chronic forms of Migraine. The article talks about several devices that are extensively used in Migraine treatment (some of them are OTC devices as well). Therefore, Neuromodulation Devices used in the treatment of Migraine fulfil the gaps that can be observed in the first-line treatment, making it a viable option for patient use without any adverse complications.
Downloads
Article in PDF
Recent Articles
- Rapid Medical’s TIGERTRIEVER 13; Glaukos’s Istent Infinite System; GE Healthcare’s Definium 656 H...
- Continuous R&D Ramps Up the Pacemakers Pipeline Landscape, New Devices To Enter the Market Soon
- GE HealthCare, DePuy Synthes Announced Collaboration; Eko Health Launched CORE 500 Digital Stetho...
- QuantuMDx & Menarini’s Agreement; TruClear System Launched by Medtronic in India; FDA 510(k)...
- NeuroLogica’s OmniTom Elite; Cordis’s S.M.A.R.T. RADIANZ Vascular Stent System; Rockley Photonics...



