New Approach Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitors
Aug 10, 2015
Introduction:
Cardiovascular disease is the leading cause of mortality worldwide due to increased level of Low Density Lipoprotein, cholesterol, which is a major modifiable risk factor. One of the risk factors is atherosclerosis caused when high levels of LDL-C in the blood build up in the inner walls of arteries, thickening them and provoking an inflammatory response, it can lead to heart attack or stroke. Rising of LDL-C are often a surrogate marker for the risk of having a cardiovascular event. Statins are in the market for almost 30 years. They are the first-line drugs for lowering cholesterol and cardiovascular events. They’ve been shown to prevent repeat heart attacks in people who have already had one and first heart attacks in a wide range of at-risk individuals. They lower LDL-C levels by inhibiting the enzyme HMG-CoA reductase, which has a vital role in the production of cholesterol in the liver. Statins typically reduce LDL-C levels by 30 to 40% and are directly associated with reducing the risk of heart attack and stroke. However, not all patients receiving statins achieve guideline-recommended low density lipoprotein (LDL) cholesterol goals, particularly those at high risk. There remains, therefore, an unmet medical need to develop additional well-tolerated and effective agents to lower LDL cholesterol levels.
The discovery of proprotein convertase subtilisin/kexin type 9 (PCSK9), a secretory protein that posttranscriptional regulates levels of low density lipoprotein receptor (LDLR) by inducing its degradation, has opened a new era of pharmacological modulation of cholesterol homeostasis. PCSK9 is therefore a novel target for lipid-lowering therapy. Inhibition of PCSK9 acts synergistically with existing treatments such as statins. PCSK9 is a crucial protein in LDL cholesterol (LDL-C) metabolism, by virtue of its pivotal role in the degradation of the LDL receptor.
Downloads
Article in PDF
Recent Articles
- Invokana matches; Xultophy beats; Lilly and BI follow; Tresiba matches
- Sanofi invests; ICER pitches; $6M in NIH; Pfizer, Roche cancer drug pricing
- Business Cocktail
- Novartis on AMD drug; Hospira recall of vials; Takeda wraps up; FDA bans imports
- Ocugen-Bharat Biotech’s Covaxin Against B.1.617 Corona Variant; uniQure Shares; J&J Covid Va...
Major advantages of developing PCSK9 inhibitors:
The discovery of the PCSK9 inhibitors and its role in LDL metabolism provides a clear example of the power of modern techniques in genetics and genomics and the current rapidity of translational science. Basic science data on the biology of PCSK9 made it clear that PCSK9 possesses many desirable attributes of a potential drug target. Statins work indirectly to increase LDL receptors via inhibition of cholesterol synthesis, a process that occurs in all tissues and the consequences of which may help explain the 5% to 20% rate of statin intolerance. In this way, fewer off-target effects could favorably distinguish PCSK9 inhibitors from statins. A second desirable trait is afore knowledge that inhibition or loss of function of the target is likely to have the desired effect and be safe in humans.
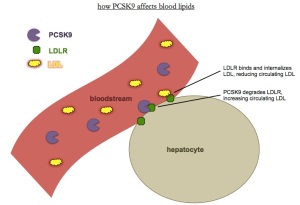
Several mechanisms of drug action for the inhibition of PCSK9 have been proposed. Specific antibodies against PCSK9 appear to be a promising drug development modality. According to recently published market report in FierceBiotech, the PCSK9 market could be worth $10 billion a year. Even recent worries from the FDA about potential neurocognitive side effects, which initially hit Regeneron and Sanofi shares hard, have eased, at least for now.
Safety profile of PCSK9 inhibitors:
No severe or life-threatening adverse events have been attributed to the PCSK9 inhibitors. Common side effects include injection site reactions, and cold and flu-like symptoms. However, Amgen and Regeneron/Sanofi are assessing potential neurocognitive side effects, such as memory loss and confusion. And Kastelein says it is possible that the body’s own antibodies to the monoclonal antibody treatments could cause rare problems over the long term something that will emerge through continued surveillance once the treatments reach market.
The future of PCSK9 inhibitors:
Statins have become the cornerstone of dyslipidemia management over the past 30 years. However, there are still two major unfilled gaps with statin use. Some patients, especially patients with Familial Hypercholesterolemia (FH), fail to achieve their LDL-C target at the maximally tolerated dose of statin, even when combined with other medications. Residual cardiovascular risk remains even with LDL-C levels < 70 mg/dL. So the development of the PCSK9 inhibitors could be an advantageous treatment strategy to fill these gaps.
PCSK9 inhibitors are expected to work synergistically with statins. The increased level of PCSK9 reduces the effect of statins by enhancing the degradation of LDL receptors. This finding suggests that the combination of a statin with a drug inhibiting PCSK9 activity could achieve a greater LDL-C reduction than statin alone.
Development of PCSK9 Inhibitors
There are 25+ pipeline drugs with 17+ companies actively involved in drug development. There is 1 drug in phase III, 2 in phase II, 6 in phase I, 8 in pre-clinical and 3 in discovery. Amgen was the first to seek regulatory approval for a PCSK9 inhibitor with its application to the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) for Evolocumab in August and September 2014, respectively. But Regeneron/Sanofi could yet beat Amgen to it. The marketing authorization application for Alirocumab was accepted by the EMA and FDA in January 2015, but the FDA has agreed to speed up the process by selecting it for priority review, aiming for a six-month turnaround. Hot on their heels is Pfizer, whose PCSK9 inhibitor Bococizumab (RN316) is currently in phase III trials.
Conclusion:
From the data currently available, it is conceivable to conclude that, due to its function as a regulator of LDLR protein expression in the liver, PCSK9 represents the most promising pharmacological target for the new era of treatment of cardiovascular diseases. An important effort has been made in order to develop new PCSK9 inhibitors and in the future, therapies capable to inhibit PCSK9 expression, processing, and interaction with LDLR (mAbs) could represent a real benefit for the treatment of hypercholesterolemia and associated cardiovascular diseases.
This new line of therapy to hyperlipidemia is a boon, especially to the FH and statin resistant population whooping large amounts to the industry. With the growing need of these drugs as maintainace therapy for years and the tight regulations for biologic, these PCSK9 inhibitors are going to earn billions for years in the absence of generic competition.
DelveInsight’s, Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitors – Pipeline Insights, 2015 Report:
The report provides the in-depth analysis of the pipeline assets across the PCSK9 inhibitors. The Report gives insights on 25+ products with 7+ different technologies. It also includes around 15+ companies which are active in this field. DelveInsight’s, Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitors – Pipeline Insights, 2015 Report covers PCSK9 inhibitors pipeline molecules at various stages of development like Pre-registration phase, clinical phases (Phase III, Phase II & Phase I), pre-clinical and discovery phases. The Report also provides PCSK9 inhibitors related therapeutic assessments by molecule type, route of administration, monotherapy and combination products. The Report also highlights the discontinued and inactive projects in pipeline for PCSK9 inhibitors.
This Report will help you to identify emerging players with potentially strong product information, licensing opportunities and helps you to create effective counter-strategies to gain competitive advantage.
For more information on Pipeline Insight Reports, email at info@delveInsight.com
Downloads
Article in PDF
Recent Articles
- Letter for AstraZeneca; Patheon on API plant; Sanofi, Regeneron’s Dupixent; Cerulean Pharma and D...
- Can Dupixent Be A Gamechanger In The Atopic Dermatitis Treatment Landscape?
- Business Cocktail
- Notizia
- Cost-effectiveness, Advanced Technology, Rising Demand Pushes the Insulin Delivery Devices Market



