Ipsen’s Onivyde Combo Ends a Decade-Long Dry Spell in Newly Diagnosed Pancreatic Cancer
Feb 23, 2024
It marks the awaited green light for Ipsen following its acquisition of Onivyde in 2017. The FDA has given its nod to the supplemental new drug application for Onivyde (irinotecan liposome injection) in combination with oxaliplatin, fluorouracil, and leucovorin (NALIRIFOX) as a primary treatment for adults with metastatic pancreatic adenocarcinoma (mPDAC). This approval represents the second approval for an Onivyde treatment regimen in mPDAC, building upon the FDA’s 2015 approval of Onivyde alongside fluorouracil and leucovorin after progression on gemcitabine-based therapy.
Julie Fleshman, CEO of the Pancreatic Cancer Action Network, stated that Nalirifox represents a significant milestone as the first newly approved treatment regimen tailored specifically for initial therapy of pancreatic cancer in over a decade. This innovative approach combines Onivyde with the chemotherapy drugs oxaliplatin and fluorouracil, along with leucovorin. Essentially, Onivyde, also known as liposomal irinotecan, substitutes the conventional form of irinotecan found in the previous Folfirinox regimen.
Pancreatic ductal adenocarcinoma (PDAC) stands as the prevalent form of pancreatic cancer, affecting over 60,000 individuals annually in the United States and close to 500,000 worldwide. Typically asymptomatic in its initial stages, PDAC often goes undetected until it reaches an advanced stage, characterized by its metastasis to other organs (stage IV). Symptoms such as weight loss, abdominal discomfort, and jaundice emerge as the disease progresses, complicating its early identification. Despite considerable advancements in cancer therapy since the 1970s, PDAC remains largely resistant to available treatments, failing to significantly prolong patients’ lives. Presently, less than 20% of PDAC patients survive beyond one-year post-diagnosis. Globally and in the US, pancreatic cancer records the lowest five-year survival rate among all cancer types.
Downloads
Article in PDF
Recent Articles
- Treatment to Clinical Development – A Bumpy Journey for Pancreatic Cancer
- Gastrointestinal Cancers: Exploring the Range of Digestive Tract Malignancies
- FDA Approves RINVOQ for Crohn’s Disease; FDA Approves Krystal Biotech’s Gene Therapy Vyjuve...
- Antibody–Drug Conjugates: An Emerging Concept in Cancer Therapy
- Pancreatic Cancer Treatment to Stride Beyond Chemotherapy
Dr. Zev Wainberg, Professor of Medicine and Co-Director of the UCLA GI Oncology Program, remarked that managing metastatic pancreatic adenocarcinoma presents significant challenges due to the limited treatment options available. He emphasized the importance of advancements in the treatment landscape, stating that each progress represents a meaningful enhancement in patient outcomes given the aggressive nature and complexity of the disease. Dr. Wainberg highlighted the significance of the approval of the Onivyde regimen, citing the NAPOLI III trial’s demonstration of survival benefits compared to current standard treatment options. He noted that this approval marks an important milestone for individuals with metastatic pancreatic adenocarcinoma, their families, and healthcare providers.
Onivyde is a cancer medication designed to inhibit the activity of an enzyme known as topoisomerase I, crucial for the replication of cell DNA required for cell proliferation. By obstructing this enzyme, Onivyde impedes the multiplication of cancer cells, leading to their eventual demise. Irinotecan, the active ingredient in Onivyde, is encapsulated within minuscule lipid particles called liposomes, facilitating its accumulation within tumors and gradual release over time. The administration of Onivyde involves a 90-minute intravenous infusion every two weeks, with suggested adjustments to the dosage regimen. In the United States, Onivyde may be promptly prescribed for eligible individuals with metastatic pancreatic ductal adenocarcinoma who are either new to treatment or have previously received gemcitabine-based therapy.
The Ipsen Cares patient support program is accessible in the United States to assist eligible individuals with mPDAC and their caregivers. It offers educational assistance and helps with questions regarding coverage, access, and reimbursement to facilitate access to Onivyde.
In 2020, the FDA granted Ipsen Fast Track designation for Onivyde as a primary combination therapy for mPDAC. The FDA’s Fast Track initiative expedites the evaluation of medications targeting severe illnesses with the potential to fill medical gaps. Ipsen holds exclusive rights to market Onivyde for current and potential future uses in the US, while Servier, an international pharmaceutical firm operating in 150 countries, manages Onivyde’s commercialization globally except in the US and Taiwan. In Taiwan, PharmaEngine, an oncology company based in Taipei, oversees the commercialization of Onivyde.
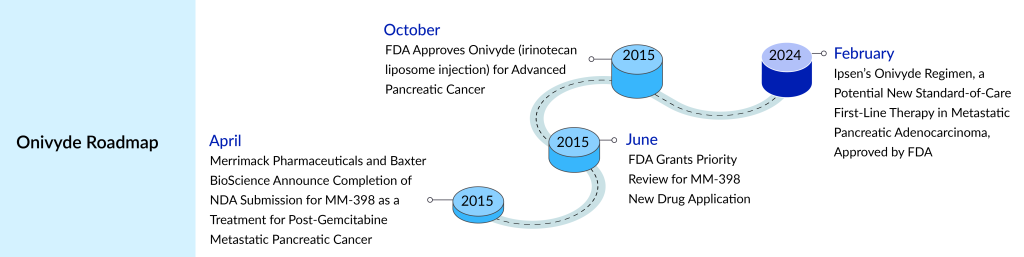
The FDA’s approval was granted after reviewing the effectiveness and safety findings from the NAPOLI III trial. NAPOLI 3, a Phase III study, was conducted openly and randomly, evaluating an investigational treatment regimen called NALIRIFOX, utilizing Onivyde, in patients with metastatic pancreatic ductal adenocarcinoma (mPDAC) who hadn’t undergone prior chemotherapy. The trial included 770 participants from 18 countries spanning Europe, North America, South America, Asia, and Australia, with enrollment across 187 trial sites. Patients were randomly assigned to either receive the NALIRIFOX regimen, consisting of Onivyde along with oxaliplatin, fluorouracil, and leucovorin, administered twice monthly (on days 1 and 15 of a 28-day cycle), or to receive nab-paclitaxel and gemcitabine injections three times monthly (on days 1, 8, and 15 of a 28-day cycle).
“We are delighted by the new approval of the NALIRIFOX regimen by the US Food and Drug Administration. Each newly approved treatment brings renewed hope for future patients and potentially offers more quality time for those currently battling pancreatic cancer alongside their loved ones,” stated Julie Fleshman, JD, MBA, President and CEO of the Pancreatic Cancer Action Network (PanCAN), a patient advocacy group dedicated to furnishing evidence-based information and support to patients and caregivers while driving forward research efforts to enhance patient outcomes. “We express our gratitude to the patients who took part in this clinical trial, as their participation is integral to the advancement of pancreatic cancer treatments.”
In the NAPOLI III trial, Nalirifox demonstrated a 16% decrease in the risk of mortality compared to the conventional chemotherapy regimen involving Bristol Myers Squibb’s Abraxane and gemcitabine. Patients treated with the Onivyde-containing protocol survived for a median duration of 11.1 months, whereas those in the control group lived for 9.2 months. Typically, a mortality risk reduction of less than 20% is not considered clinically significant in most cancer treatments. However, in the context of pancreatic cancer, the 16% enhancement with Nalirifox was both statistically significant enough to win FDA approval. Nalirifox reduced the risk of tumor advancement or mortality by 30% in comparison to the conventional chemotherapy combination. It elicited a response rate of 41.8%, which was marginally higher than the 36.2% seen in the control group.
Christelle Huguet, EVP and Head of Research and Development at Ipsen stated that the Phase III NAPOLI 3 trial’s findings mark the first positive outcomes for a new treatment regimen for metastatic pancreatic adenocarcinoma compared to the currently approved nab-paclitaxel and gemcitabine regimen. With the recent approval, the Onivyde (NALIRIFOX) regimen presents a promising new standard-of-care option, demonstrating proven survival advantages for individuals with metastatic pancreatic adenocarcinoma in the US.
Additionally, Ipsen, along with its partner Genfit, anticipates an FDA decision regarding elafibranor as a second-line therapy for primary biliary cholangitis, a rare autoimmune liver ailment, by June 10. These product launches coincide with the gradual erosion of Ipsen’s leading drug, Somatuline, due to generics. Furthermore, analysts pointed out that Onivyde itself will confront patent expiry by the close of 2027. Hence, Ipsen is banking on further business development agreements to propel the company into its next growth phase. By the end of 2023, Ipsen had 1.9 billion euros in potential dealmaking capacity.

Downloads
Article in PDF
Recent Articles
- Pancreatic Cancer Treatment to Stride Beyond Chemotherapy
- AstraZeneca’s Danicopan Trial; CHMP Recommends Sanofi/AstraZeneca’s nirsevimab; Akero’s NASH Drug...
- FDA Approves PENBRAYA for Most Common Serogroups Causing Meningococcal Disease; BIMZELX Approved ...
- Zejula’s priority review; Erytech Pharma’s new facility; good news for Krystal
- Treatment to Clinical Development – A Bumpy Journey for Pancreatic Cancer