ORLYNVAH – The First Oral Penem in the US Market: A New Era in UTI Treatment
Nov 08, 2024
Urinary tract infections (UTIs) are among the most prevalent bacterial infections in the US and globally. Around 60% of women will experience an uncomplicated UTI (uUTI) during their lifetime, and up to 40% of women with a history of uUTIs may face a recurrence, according to Iterum’s approval announcement.
In 2023, the total diagnosed cases of uncomplicated urinary tract infections in the US were 9.4 million. These cases are expected to grow with a significant CAGR in the study period 2020–2034, as per DelveInsight’s latest assessment in the “Uncomplicated Urinary Tract Infection Epidem Report”.
In the US, approximately 40 million prescriptions for uUTIs are written annually. Iterum estimates that about 1% of these infections are caused by pathogens resistant to all commonly prescribed oral antibiotics.
Downloads
Article in PDF
Recent Articles
- Samsung Partnered with Lunit; Boston Scientific Launched VersaVue single-use Flexible Cystoscope;...
- BMS, and Exelixis’s Opdivo + CABOMETYX in First-Line Advanced Renal Cell Carcinoma; AIRSUPRA Now ...
- Amgen’s Olpasiran Candidate; GSK’s Novel Antibiotic for Urinary Tract Infections; AstraZeneca and...
- Urinary Tract Infections (UTIs)– How is it affecting the Globe?
- Roche Secures FDA Clearance for High-Volume Slide Scanner; Pacira Gets 510(k) Approval for Iovera...
Current Uncomplicated UTIs Treatment Options
Currently, antibiotics are the primary treatment for urinary tract infections, as they help reduce both the severity and duration of symptoms and prevent complications. Commonly used antimicrobial agents for uncomplicated UTIs include the combination of trimethoprim and sulfamethoxazole, trimethoprim, β-lactams, fluoroquinolones, nitrofurantoin, and fosfomycin. The main aim of antimicrobial therapy is to eliminate the infecting organisms from the urinary tract and alleviate symptoms.
According to the AUA/CUA/SUFU guidelines, nitrofurantoin, TMP-SMX, and fosfomycin are recommended as first-line treatments for symptomatic UTIs in women. Trimethoprim-sulfamethoxazole has long been considered the standard therapy for both acute and recurrent UTIs due to its effectiveness against common uropathogens, as well as its affordability and good tolerability.
Pivmecillinam, a global first-line therapy for uncomplicated UTIs, is not available in the US. This absence has spurred the emergence of alternative treatments, indicating a need for approved options to address uUTIs in the country.
Entry of ORLYNVAH in the Uncomplicated UTIs Treatment Segment
Despite recent FDA concerns over potential off-label use and antimicrobial resistance, Dublin-based Iterum Therapeutics has received approval for its oral antibiotic, sulopenem. Marketed as ORLYNVAH, this is Iterum’s first FDA-approved product, specifically indicated for infections caused by Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis in women with limited or no alternative oral antibiotic options.
This approval—Iterum’s first FDA nod—is the second uUTI treatment approved this year, breaking a two-decade lull in the field. Notably, it is also the first US approval for an oral penem antibiotic. In April, the FDA approved Utility Therapeutics’ PIVYA (pivmecillinam). Although this penicillin-based antibiotic had been approved in Europe for over 40 years, it had never previously been authorized for use in the US.
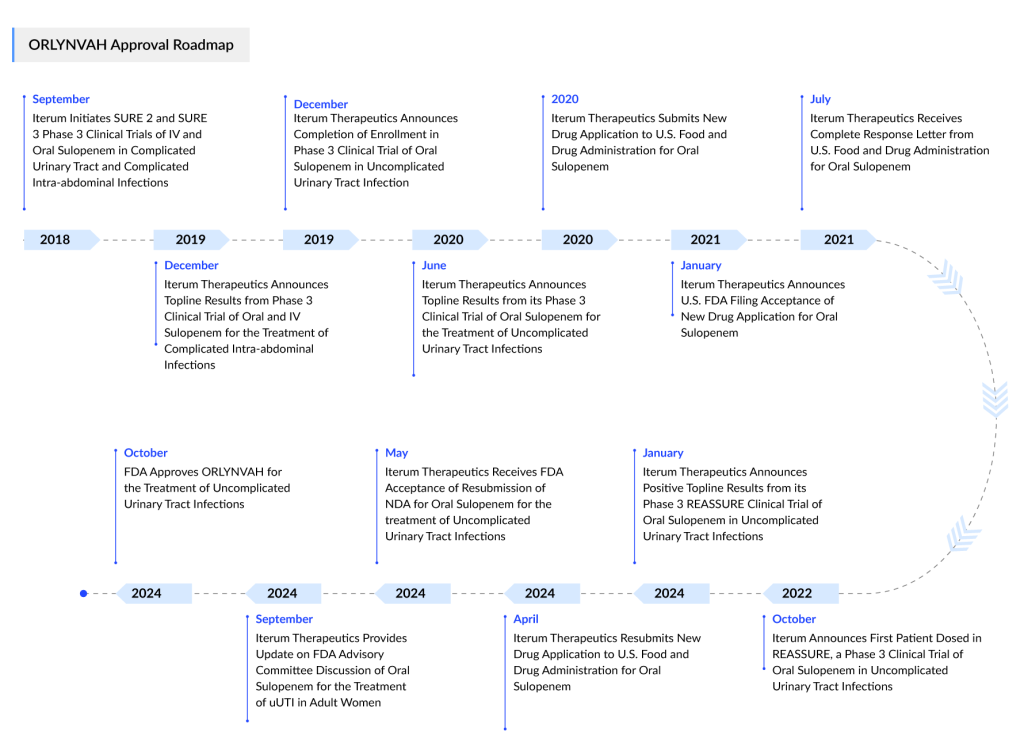
On October 25, 2024, the FDA’s decision was supported by Iterum’s clinical data for ORLYNVAH, which included two Phase III trials. In the pivotal REASSURE study, a late-stage trial with topline data released in January 2024, oral sulopenem demonstrated statistical non-inferiority to Augmentin (amoxicillin/clavulanate) in overall response. ORLYNVAH notably outperformed Augmentin in terms of overall treatment success, with similar safety profiles observed across treatment groups.
Iterum’s data also encompassed results from the Phase III SURE 1 trial. This study, published in June 2020, showed that oral sulopenem was more effective than ciprofloxacin in patients with quinolone-resistant infections. SURE 1 also confirmed ORLYNVAH’s safety, with adverse events occurring at a comparable rate to those in the ciprofloxacin group.
Although Iterum filed a New Drug Application with the FDA, approval was not granted. In July 2021, the FDA issued a Complete Response Letter to the Dublin, Ireland-based biotech, indicating that while SURE 1 had demonstrated oral sulopenem’s superiority to ciprofloxacin, Iterum would need to conduct an additional trial with a different comparator drug.
Penems are already extensively used intravenously due to their established efficacy and safety. However, patients have traditionally required treatment in hospital settings, as noted by an Iterum spokesperson in an email. ORLYNVAH, as an oral alternative to a proven antibiotic, is expected to be accessible through outpatient visits.
Future uUTI Treatment Space Looks Promising
The future of urinary tract infection treatment is brimming with promise as cutting-edge innovations reshape the landscape. With rising antibiotic resistance posing a significant challenge, the focus is shifting toward novel therapeutic approaches, including targeted antimicrobial therapies, vaccine development, and harnessing the power of microbiome modulation
Some of the drugs in the uUTI pipeline that may give tough competition to Iterum’s ORLYNVAH include GSK’s Gepotidacin (GSK2140944) and Inmunotek’s Uromune (MV140) among others. Gepotidacin (GSK2140944) is a bacterial type II topoisomerase inhibitor, classified as a triazaacenaphthylene, being developed by GlaxoSmithKline for the treatment of uncomplicated urinary tract infections. It represents the first antibiotic in a novel class developed by GSK in 2007, featuring a unique dual-target mechanism of action and an oral formulation.
Gepotidacin MoA is distinct from any currently approved antibiotic, working by specifically targeting two crucial bacterial enzymes—DNA gyrase and topoisomerase IV—that are involved in bacterial replication. The development of Gepotidacin is the result of a public-private partnership between GSK, the US government’s Biomedical Advanced Research and Development Authority (BARDA), and the Defense Threat Reduction Agency (DTRA).
Uromune is a glycerin-based suspension containing inactivated whole cells of bacteria, designed for sublingual use. It is applied by spraying onto the sublingual area and is intended for the patient to administer independently.
The expected launch of potential therapies in the market by key players during the forecast period (2024–2034), shall create a positive impact on the market size of uncomplicated urinary tract infections in the US. Moreover, a high demand exists for new drugs addressing multi-drug resistance in uncomplicated urinary tract infections due to which the uUTI market is anticipated to change in the coming years.
Additionally, these advancements are paving the way for more effective, personalized treatments that not only address the root causes of UTIs but also reduce recurrence and improve patient outcomes. As research continues to unravel new possibilities, the UTI treatment space is poised for a revolution, offering hope for better management and a brighter future for patients worldwide.

Downloads
Article in PDF
Recent Articles
- Urinary Tract Infections (UTIs)– How is it affecting the Globe?
- Uncomplicated UTI Treatment: Challenges and Opportunities in the Market
- Amgen’s Olpasiran Candidate; GSK’s Novel Antibiotic for Urinary Tract Infections; AstraZeneca and...
- BMS, and Exelixis’s Opdivo + CABOMETYX in First-Line Advanced Renal Cell Carcinoma; AIRSUPRA Now ...
- Samsung Partnered with Lunit; Boston Scientific Launched VersaVue single-use Flexible Cystoscope;...