OZEMPIC’s New Approval Cements Novo’s Lead in GLP-1 Market
Feb 03, 2025
The recent approval of OZEMPIC reinforces the growing trend of GLP-1 drugs being used for conditions beyond diabetes and weight loss. Novo Nordisk’s blockbuster GLP-1 medication, OZEMPIC, has secured another label expansion, as the FDA has now approved it for treating chronic kidney disease.
The approval for chronic kidney disease (CKD)—a first among GLP-1 receptor agonists—positions OZEMPIC as the most widely indicated drug in its class. Initially approved in 2017 for glycemic control in type 2 diabetes, OZEMPIC later received FDA approval in 2020 to reduce the risk of major adverse cardiovascular events in adults with T2D and established cardiovascular disease. The drug, known as semaglutide, further expanded its reach into weight management with its launch as WEGOVY in 2021. Last year, WEGOVY also secured approval for reducing cardiovascular risk.
Keen to know more about WEGOVY? Read our blog “WEGOVY: A Leader in the Obesity and Weight Loss Treatment” for more insights
Downloads
Article in PDF
Recent Articles
- Assessment of Key Products that Got FDA Approval in Second Half (H2) of 2021
- Breakthroughs in Alport Syndrome Treatment: A New Era of Hope
- Sky Medical’s Geko™ device; Fist Assist Devices obtains Breakthrough Device designation; E...
- AUCATZYL Approved for R/R B-ALL; FDA Accepts NDA for Unicycive’s Oxylanthanum Carbonate; AstraZen...
- 7 Promising Obesity Drugs Set to Launch by 2027
“Chronic kidney disease is a serious and prevalent condition among individuals with type 2 diabetes, posing a critical need for those managing both conditions. The approval of OZEMPIC allows us to address issues within the cardiovascular-kidney-metabolic syndrome, which impacts millions of adults and could lead to severe outcomes if not treated,” stated Anna Windle, PhD, Senior Vice President of Clinical Development, Medical & Regulatory Affairs at Novo Nordisk. “With this new approval, OZEMPIC is now recognized as the most broadly approved GLP-1 RA in its category. We are proud to continue advancing innovations that will positively impact this patient population, reinforcing Novo Nordisk’s dedication to cardiometabolic care.”
Novo estimates that chronic kidney disease impacts approximately 37 million adults in the US, and this figure is expected to grow in the future. Additionally, CKD is a frequent complication of Type 2 diabetes, affecting roughly 40% of individuals with the condition. The estimated total diagnosed prevalent cases of CKD in the US were nearly 5 million in 2023, as per the latest assessment on Chronic Kidney Disease Epidem-based Market Forecast Report.
OZEMPIC injection, available in 0.5 mg, 1 mg, or 2 mg doses, is an injectable prescription medicine used along with diet and exercise to improve blood sugar in adults with type 2 diabetes. It also reduces the risk of major cardiovascular events, such as heart attack, stroke, or death, in adults with type 2 diabetes and known heart disease, and decreases the risk of kidney disease worsening, kidney failure (end-stage kidney disease), and death due to cardiovascular disease in adults with type 2 diabetes and chronic kidney disease. The safety and effectiveness of OZEMPIC in children have not been established.
This FDA approval of OZEMPIC is based on the outcomes of the FLOW phase IIIb kidney trial, which explored the impact of once-weekly OZEMPIC injections on major kidney and cardiovascular outcomes in adults with type 2 diabetes and chronic kidney disease. The FLOW trial met its primary endpoint, showing that OZEMPIC 1 mg led to a significant 24% relative risk reduction in the worsening of kidney disease, kidney failure, and death from cardiovascular disease (4.9% absolute risk reduction at 3 years) when compared to a placebo, in addition to standard care.
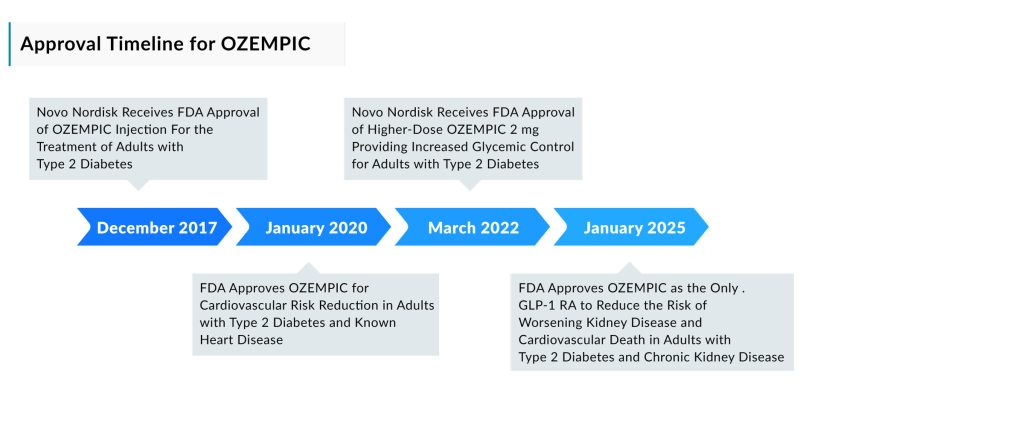
The FLOW trial was an international, randomized, double-blind, parallel-group, placebo-controlled study designed to assess the effectiveness of once-weekly OZEMPIC 1 mg versus placebo, in addition to standard care, on kidney outcomes in adults with type 2 diabetes and chronic kidney disease. The goal was to reduce the occurrence of a primary composite endpoint that included a sustained decline in eGFR of ≥50%, sustained eGFR < 15 mL/min/1.73 m², chronic renal replacement therapy, renal death, and cardiovascular death. The trial involved 3,533 participants (1,767 receiving OZEMPIC and 1,766 receiving placebo) across 28 countries at around 400 clinical sites. Initiated in 2019, the study was halted early based on a recommendation from the Independent Data Monitoring Committee after a median follow-up of 3.4 years, as it met the pre-defined efficacy criteria.
“Managing type 2 diabetes can already be difficult, and the added complication of chronic kidney disease only makes it more challenging. From my experience, patients with both conditions require additional support through medications that can significantly reduce the risk of major kidney and cardiovascular issues,” said Richard E. Pratley, MD, Medical Director at the AdventHealth Diabetes Institute in Orlando, FL, and Co-Chair of the FLOW Trial. “Many of the patients I see suffer from severe kidney complications and comorbidities, with some needing dialysis. The FDA’s decision today brings hope to the millions of adults living with both conditions, offering a new treatment option that represents a major advancement for my patients.”
The label expansion for OZEMPIC could help Novo stay ahead in the increasingly competitive obesity market, which has recently seen a surge in clinical developments. On 27 January 2025, Veru Inc. released Phase IIb data for its experimental selective androgen receptor modulator, highlighting a 71% reduction in lean mass loss in seniors using WEGOVY. The same day, the emerging obesity company Metsera launched a $250 million IPO, targeting a $1.78 billion valuation. Then on 28 January 2025, Versant Ventures introduced Helicore Biopharma—marking the investor’s fourth obesity-focused biotech in a year and the second new company in the field this month.
Eli Lilly, a competitor in the GLP-1 market, is also aiming to expand the use of its tirzepatide, which was approved for type 2 diabetes in 2021 under the brand name MOUNJARO and for weight loss as ZEPBOUND in late 2023. In December 2024, the FDA approved ZEPBOUND as the first-ever treatment for obstructive sleep apnea. These two leaders in weight loss aren’t the only ones pursuing additional indications for GLP-1 drugs.
Track ZEPBOUND journey in OSA at Lilly’s ZEPBOUND Clears Hurdle in Sleep Apnea Treatment
Novo’s recent approval comes as its semaglutide portfolio—encompassing OZEMPIC and Rybelsus for diabetes, and WEGOVY for obesity—continues to perform well.
In addition to generating billions in annual revenue, OZEMPIC and WEGOVY—both of which gained significant attention for their weight loss benefits—have largely avoided the supply shortages that have affected them for years.
At the same time, Novo is not resting on its success, as it explores the use of semaglutide for various other conditions, including metabolic dysfunction-associated steatohepatitis, heart failure, and even Alzheimer’s disease.

Downloads
Article in PDF
Recent Articles
- 7 Promising Obesity Drugs Set to Launch by 2027
- Assessment of Key Products that Got FDA Approval in Second Half (H2) of 2021
- Beyond Supportive Care: How New Drugs are Shaping Metabolic-associated Steatohepatitis (MASH) Tre...
- 8 Emerging Obesity Trends Transforming Therapeutics Segment
- Autologous cell therapy market: A new paradigm for kidney diseases