Feb 07, 2024
Navigating the Healthcare Horizon: Odyssey of Mergers, Funding, and Acquisitions in 2024
As we step into the crisp corridors of 2024, the healthcare landscape unfolds a compelling saga of mergers, strategic funding, and transformative acquisitions. In this month-by-month analysis, we delve into the intricate tapestry of industry dynamics, exploring the impactful maneuvers that are shaping the healthcar...
Read More...
Jun 27, 2023
FDA Approves Jardiance for Type 2 Diabetes; FDA Approves Pfizer’s LITFULO for Alopecia Areata; Sarepta Therapeutics’s ELEVIDYS Approval; Tonix Pharmaceuticals to Acquire Two Migraine Products from Upsher-Smith; FibroGen’s Phase 3 ZEPHYRUS-1 Study of Pamrevlumab; FDA Orphan Drug Designation to ERAS-801 for Malignant Glioma
FDA Approves Jardiance for the Treatment of Type 2 Diabetes in Children 10 Years and Older Boehringer Ingelheim and Eli Lilly and Company announced that the FDA has approved Jardiance® (empagliflozin) 10 mg and 25 mg tablets to decrease blood sugar together with diet and exercise in children 10 years and older w...
Read More...
Nov 21, 2022
RNA Interference: New Class of Drugs In The Fight Against Disease
RNA interference (RNAi) is a fundamental gene-silencing pathway in eukaryotic cells that involves an enzyme called dicer cleaving long pieces of double-stranded RNA into shorter fragments called siRNAs that can cleave complementary mRNA sequences with the help of the RISC complex and argonaute. RNA interference ...
Read More...
Nov 01, 2022
Actinium Announces SIERRA Trial Results; Santhera Seeks FDA Review for Vamorolone; Seres Announces BLA Submission for SER-109; BMS Announces Results of COMMANDS Trial; Boehringer’s PDE4B Moves Late-stage Clinical Testing; FDA Rejects Gilead’s Hepcludex; Approval to J&J’s BCMAxCD3 Bispecific Antibody for Multiple Myeloma; Syncona to Acquire AGTC
Actinium Announces Positive Top-line Results from Pivotal Phase III SIERRA Trial of Iomab-B Actinium Pharma is on track to submit its targeted radiotherapy for AML patients requiring a bone marrow transplant in the United States, boosted by top-line data from a pivotal trial. The SIERRA trial of Iomab-B, an anti...
Read More...
Oct 25, 2022
Sumitomo to Purchase Myovant; AstraZeneca’s Tremelimumab Plus Imfinzi Approved in the US; AbbVie Acquires DJS Antibodies; Roche and Hookipa Pharma Signs USD 25 Million Deal; FDA Accepts BMS’s New Drug Application for CAMZYOS; Jazz and Zymeworks Sign Exclusive Licencing Agreement
FDA Accepts Bristol Myers Squibb’s Supplemental New Drug Application for CAMZYOS Bristol Myers Squibb declared that the U.S. FDA had accepted its supplemental new drug application for CAMZYOS for an expanded indication to reduce the need for septal reduction therapy. CAMYZOS is currently FDA-approved for treatin...
Read More...
May 17, 2022
BMS Sells NY Biologics Plant; FDA Approves Mitsubishi Tanabe’s Radicava, Love Pharma Acquires MicroDoz; Sandoz Launches Generic of Roche’s Esbriet; Pfizer to Acquire Biohaven Pharma; eureKARE’s Cell and Gene Therapy CDMO; Atamyo’s LGMD Gene Therapies; FDA approves Eli Lilly’s Type 2 Diabetes Treatment
Love Pharma Completes the Acquisition of MicroDoz Therapy LOVE Pharma Co. has announced the completion of its acquisition of MicroDoz Theraphy Inc. ("MicroDoz"). Love Pharma holds exclusive manufacturing, marketing, packaging, selling, and distribution licenses in Europe, the United Kingdom, and North America. U...
Read More...
Apr 26, 2022
Coherus Biosciences’ Toripalimab; Keymed’s CMG901; Biogen Alzheimer’s Drug Aduhelm; Vicore’s Digital Therapeutics Study; Cassiopea’s Acne Treatment Winlevi; Samsung Biologics Acquires Samsung Bioepis; Roche’s Giredestrant; Ashai Kasei Acquires Bionova
FDA Grants Orphan Drug Designation to Coherus Biosciences’ Toripalimab for Small Cell Lung Cancer The FDA granted orphan drug status to Coherus Biosciences’ Toripalimab (TuoYi) based on data from the phase 3 JUPITER-08 study in patients with extensive-stage Small-cell Lung Cancer (ES-SCLC). Patients in the curre...
Read More...
Jan 18, 2022
Algernon’s NP-120 (ifenprodil); Pieris’s cinrebafusp alfa (PRS-343) clinical trial; Gaumard Scientific’s multidisciplinary patient simulator HAL S5301; Hekka Labs’s decentralized healthcare ecosystem
Algernon receives positive FDA feedback on Phase IIb chronic cough trial The US Food and Drug Administration (FDA) has given positive feedback for Algernon Pharmaceuticals’ Phase IIb clinical trial of NP-120 (ifenprodil) for chronic cough treatment. A receptor antagonist of N-methyl-D-aspartate (NMDA), ifenp...
Read More...
Aug 10, 2020
Boehringer Ingelheim; Roche demonstrated new analyses of their drugs in patients with ILDs during the American Thoracic Society (ATS) Virtual conference
ATS 2020 Virtual is scheduled to be held from August 5 to August 10, and it is exciting to watch the key presentations by Pharmaceutical Companies specifically focused on pulmonary disease, critical illness, and sleep disorders. Boehringer Ingelheim presented new analyses of Ofev data in patients with chronic fi...
Read More...
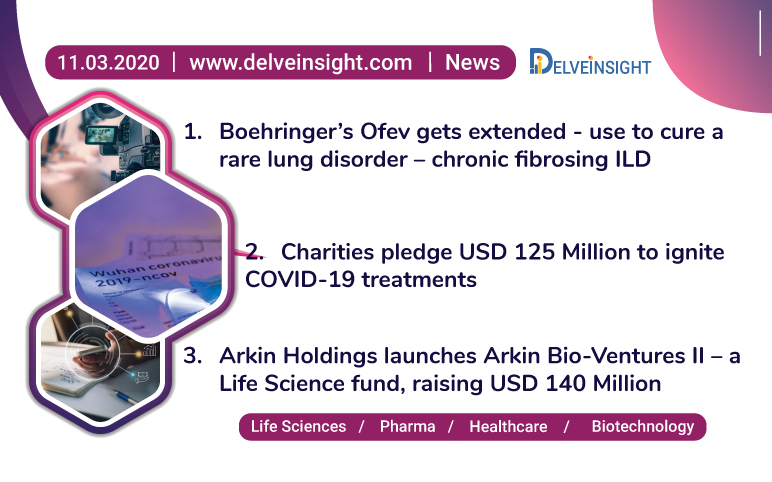
Mar 11, 2020
Ofev’s expanded use; Arkin Bio-Ventures II launch; USD 125 M for COVID-19 treatment
Boehringer Ingelheim’s Ofev (nintedanib) has received FDA recommendation for the treatment of chronic fibrosing interstitial lung diseases (ILD) with a progressive phenotype Interstitial Lung Disease - ILD – a group of a large number of lung disorders resulting in scarring or fibrosis of lungs, caused by conditi...
Read More...








-Agonist.png)

