Pompe Disease Treatment Landscape
Sep 30, 2024
An orphan disease is any disease that affects only a small percentage of the population. As many as 7,000 rare diseases affect human life. The total number of Americans living with a rare disease is estimated at between 25 and 30 million. In the EU, rare disease patients comprise 6% to 8 % of the population.
One such disease is Pompe disease, with an overall worldwide incidence estimated to be 1 in 40,000 live births. According to the US National Library of Medicine National Institutes of Health, the Pompe disease prevalence is approximately 1 in 28,000 in the United States. Pompe disease is mainly caused by pathogenic variations in the acid alpha-glucosidase (GAA) gene.
Additionally, more than 500 different variations of the same gene have been identified in the families living with Pompe disease. The gene is required for an efficient breakdown of glycogen, required to produce energy. Mutations in the gene cause failure in doing so, thus resulting in accrual of sugars in heart and liver.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Advances in Pompe Disease Treatment: From ERT to New Therapies
- The Transforming Pompe Disease Treatment Landscape in Emerging Markets
- Top 12 Most Expensive Drugs in the US Healthcare Market
- Unveiling Lysosomal Storage Disorders: Exploring Rare Diseases Impacting Millions Worldwide
- BeiGene’s Brukinsa Approval; FDA Approval to Seagen’s TUKYSA; NICE Recommends Alnylam’s Amvuttra;...
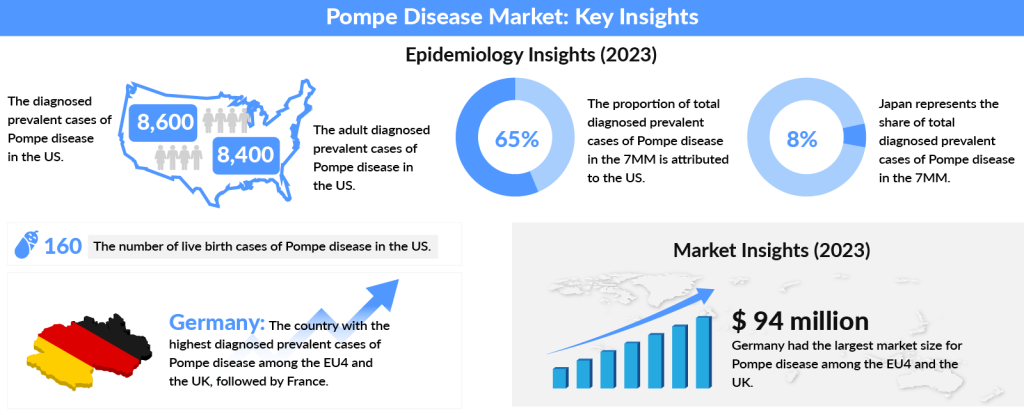
In 2023, the US represented nearly 65% of the diagnosed Pompe disease cases across the 7MM, with the number expected to rise by 2034. The US had about 8,400 adult cases, while Spain had the fewest cases. Among EU4 and the UK, Germany had the highest number of diagnosed cases, followed by France. Japan accounted for approximately 8% of the total diagnosed cases in the 7MM in 2023.
The Pompe disease therapies available are disease-specific, symptomatic, and supportive. At present, there exists no cure for treating Pompe disease. Genzyme, a Sanofi Corporation, Enzyme replacement therapy (ERT), which holds the title of the first-ever approved treatment for Pompe disease, is not a cure but can only slow down the progression. It involves the intravenous administration of recombinant human acid α-glucosidase.
In early 2022, Europe saw a significant milestone with the approval of NEXVIAZYME (avalglucosidase alfa) for the treatment of Pompe disease. This enzyme replacement therapy quickly became a beacon of hope for patients, offering a new line of defense against the debilitating effects of the disease. Fast forward to February 2024, new data revealed that NEXVIAZYME was making a tangible difference, particularly in pediatric patients with infantile-onset Pompe disease (IOPD). Over nearly three years, the therapy demonstrated notable improvements in ptosis, or drooping eyelids, highlighting its efficacy in managing a key symptom of IOPD.
The momentum didn’t stop there. September 2023 marked another milestone in the treatment landscape with the FDA’s approval of POMBILITI (cipaglucosidase alfa-atga) in combination with OPFOLDA™ (miglustat). This pioneering two-component therapy represents a significant advancement by combining POMBILITI, a bis-M6P-enriched recombinant human acid α-glucosidase (rhGAA), with OPFOLDA, an oral enzyme stabilizer. The synergy between these components aims to enhance enzyme uptake and stability, offering patients a more robust and sustained treatment option.
Pompe Disease Pipeline
Amid these developments, companies like Amicus Therapeutics and Actus Therapeutics are continuously working on developing drugs to enhance the Pompe disease pipeline and cure it from its roots. Actus Therapeutics is making strides with its promising candidate, ACTUS-101. This adeno-associated virus (AAV) gene therapy, administered intravenously, is designed to specifically target the liver and boost the production of the GAA enzyme. Although still in the early stages of development, with the first patient for the Phase I/II trial recently dosed, ACTUS-101 represents an exciting potential breakthrough in gene therapy for Pompe disease.
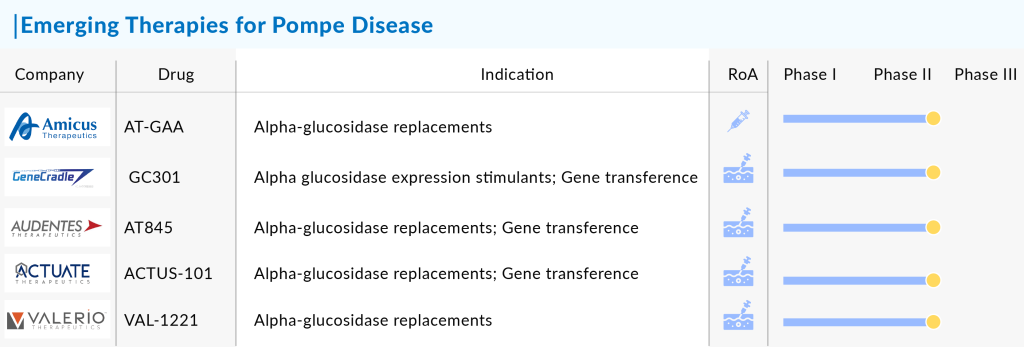
Amicus Therapeutics is also at the forefront of innovation in Pompe disease treatment with its promising clinical trials for AT-GAA, a cutting-edge fixed-dose combination therapy. This therapy, a combination of ATB200 and AT2221, represents a unique approach in treating Pompe disease. ATB200 is a recombinant human acid alpha-glucosidase (rhGAA) enzyme with optimized carbohydrate structures, particularly mannose-6 phosphate (M6P), designed to enhance cellular uptake. It is co-administered with AT2221, a pharmacological chaperone that increases the enzyme’s target specificity and overall effectiveness. Currently in Phase III of development, AT-GAA is poised to capture a significant share of the Pompe disease market upon approval due to its innovative use of chaperone technology, which aims to improve treatment outcomes by addressing the disease’s underlying pathology more precisely.
The journey of AT-GAA has been marked by notable progress. In February 2023, Amicus Therapeutics announced positive results from the global Phase 3 open-label extension (OLE) study, known as ATB200-07. This ongoing study focuses on the long-term efficacy and safety of AT-GAA in adults with late-onset Pompe disease. The study’s findings were promising: participants who received AT-GAA for up to 104 weeks demonstrated persistent and durable improvements in key clinical outcomes. These included enhanced six-minute walk distance (6MWD), stable forced vital capacity (FVC), and continued reductions in biomarkers associated with muscle damage and disease substrate. This ongoing research underscores the potential of AT-GAA to significantly impact the management of Pompe disease, offering renewed hope for patients and marking a significant step forward in the field.
Other than these, a host of innovative companies are making strides with their promising pipelines. Spark Therapeutics is on the cutting edge with its gene therapy candidate SPK-3006, which holds the potential to alter the treatment landscape for this rare condition significantly. Similarly, Astellas Pharma is making waves with AT845, a therapy that’s generating considerable excitement in the research community.
But the innovation doesn’t stop there. Big players like Genzyme, Sanofi, Amicus Therapeutics, Actus Therapeutics, Valerion Therapeutics, Astellas Therapeutics, Roche, and Lacerta Therapeutics, among others, are all contributing to the growing arsenal of potential therapies for Pompe disease.
With so many companies bringing their A-game to the table, the future of Pompe disease treatment looks incredibly promising. Each of these players is working tirelessly to turn their breakthroughs into viable treatments, offering hope to those affected by this challenging condition.
The increase in awareness about Pompe disease and support from various organizations have assured many companies that they can carry forward their research in treating and developing treatments for Pompe disease., Researchers from all over the World have made significant progress in diagnosing, treating and even finding ways to prevent rare diseases. But, even so, there is much more required to be done in the area. Many organizations, such as the FDA and the National Institutes of Health (NIH), are supporting and funding research to help improve the rare disease patient pool.
As these treatments continue to evolve, they offer renewed hope and optimism for individuals affected by Pompe disease. The rapid advancements in research and therapy not only underscore the dedication of the scientific community but also mark a transformative period in the management of this challenging condition. With each new development, patients are one step closer to a future where Pompe disease can be effectively managed, if not fully conquered.

Downloads
Article in PDF
Recent Articles
- Pompe Disease: Causes, Symptoms, Diagnosis, Pathology, Current Marketed and Emerging Drugs in the...
- Top 12 Most Expensive Drugs in the US Healthcare Market
- Avrobio taps Magenta’s ADC; Eli Lilly ramps up; FDA postpones decision; CSL gears up to fight pan...
- Navigating Challenges in Pompe Disease Treatment and Research in Eastern Europe
- Immix Bio’s NXC-201 Gets FDA RMAT for AL Amyloidosis; Biodexa’s eRapa Wins Fast Track for Familia...