Can Novo’s Rivfloza Outperform Its Rival Alnylam’s Oxlumo in Primary Hyperoxaluria Treatment Space?
Oct 09, 2023
In recent years, Novo Nordisk has been actively addressing the challenges arising from the launch of its highly popular obesity therapy, Wegovy. However, the pharmaceutical company is now venturing into a less common medical territory with its latest FDA approval. Novo has received FDA approval for Rivfloza, a treatment designed for the rare disease known as primary hyperoxaluria type 1 (PH1). This announcement comes as Novo Nordisk continues to make strides in the field of RNA interference (RNAi) drugs, with Rivfloza being a monthly subcutaneous treatment.
Following the FDA’s approval of Alnylam’s RNAi therapy Oxlumo in November 2020, this marks the second FDA-approved treatment for the rare and severe disease known as primary hyperoxaluria type 1. Primary hyperoxaluria type 1 is characterized by an excessive production of oxalate, resulting in the formation of kidney stones and a gradual decline in kidney function. It is estimated that the total prevalent population of primary hyperoxaluria in 7MM was ~12K in 2021, which is expected to increase at a CAGR of 0.66%, as per DelveInsight. The estimates suggest a higher diagnosed prevalence of primary hyperoxaluria type 1 throughout the 7MM. In the US, out of the total diagnosed prevalent cases of primary hyperoxaluria; 1,629 were occupied by primary hyperoxaluria type 1 alone, in the year 2021.
Novo Nordisk’s groundbreaking RNAi therapeutic, Rivfloza, is the product of their exclusive GalXC RNAi technology platform. Rivfloza is engineered to suppress the activity of lactate dehydrogenase, a liver enzyme crucial in the glyoxylate metabolism pathway. This inhibition aims to curb oxalate overproduction, a concern for patients with PH1. The drug’s development was spearheaded by Dicerna Pharmaceuticals, Inc., which became part of Novo Nordisk through an acquisition in 2021.
Downloads
Article in PDF
Recent Articles
- Sarepta’s deal; Fund raisings for Apexigen; Novo funded rare disease startup
- Merck to Acquire Harpoon Therapeutics; Novo Nordisk Enters Into Collaborations with Omega Therape...
- Clinical
- Primary Hyperoxaluria Market Size Observes Growth with a Substantial CAGR
- Albireo Pharma’s PFIC Therapy; Lupin’s Trichomoniasis Oral Drug; Moderna’s Weapon Aga...
Dicerna had previously investigated the drug in different types of primary hyperoxaluria (PH2 and 3) but eventually shifted its attention exclusively to PH1 based on early data. The FDA has approved Rivfloza for the reduction of urinary oxalate levels in PH1 patients aged 9 and above who still have reasonably healthy kidney function. In a phase II trial, patients on the medication showed a decrease in urinary oxalate excretion when compared to those on a placebo, as reported by Novo. Meanwhile, Alnylam, a competing company, has been actively engaged in promoting Oxlumo since its approval in 2020. By the end of June, over 350 patients worldwide were using the drug commercially, and in the second quarter, it generated $24.2 million in revenue.
“Rivfloza’s FDA approval marks another milestone in Novo Nordisk’s longstanding commitment to advancing research, fostering innovation, and forming strategic collaborations to expand treatment options for rare diseases,” commented Blandine Lacroix, Senior Vice President of Strategy and Rare Disease at Novo Nordisk Inc. “Our dedication to driving positive change for individuals living with rare diseases and addressing the considerable unmet needs of the PH1 community remains unwavering. We eagerly anticipate the availability of our first RNAi treatment for those with PH1, as well as the healthcare professionals who partner in their care.”
This approval is granted based on data obtained from the pivotal phase II PHYOX clinical trial and interim findings from the ongoing phase III PHYOX extension study. PHYOX successfully achieved its primary objective, demonstrating a significant reduction in 24-hour urinary oxalate (Uox) excretion in patients treated with Rivfloza from Day 90 to Day 180. The percentage change from baseline in 24-hour Uox was assessed through the area under the curve (AUC) analysis. The least-squares (LS) mean difference in AUC24-hour Uox between the Rivfloza and placebo groups was 4976 (95% CI: 2803, 7149; p<0.0001), signifying a significant difference over the 90-day period. The most common adverse reaction, reported in more than 20% of patients, was injection site reactions. Interim results from the PHYOX extension study demonstrate that the reductions in 24-hour Uox excretion were sustained in the 13 PH1 patients who received an additional six months of Rivfloza treatment. Novo Nordisk anticipates making Rivfloza accessible to eligible patients as early as 2024.
“RNA interference is a tried-and-true therapy option for people with PH1. With the approval of Rivfloza, we now have a novel treatment that reduces oxalate production in a safe and effective manner,” stated Dr. David S. Goldfarb, MD, Clinical Chief, Nephrology division, NYU Langone Medical Center and Professor of Medicine and Physiology, NYU Grossman School of Medicine. “Using the GalXC RNAi platform, Rivfloza targets the liver-specific lactate dehydrogenase enzyme, which is the final step of oxalate production in PH1.”
Several other companies across the globe are also diligently working toward the development of novel primary hyperoxaluria treatment therapies with a considerable amount of success over the years. Key players, such as Allena Pharmaceuticals (ALLN-177), Biocodex (Stiripentol), and others, are developing therapies for primary hyperoxaluria treatment.
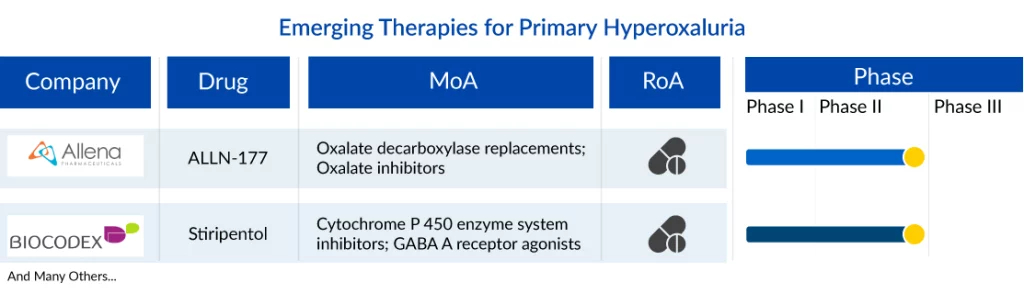
ALLN-177 is a crystalline formulation of the enzyme oxalate decarboxylase to specifically degrade oxalate within the GI tract, allowing for its removal from the body through the bowel. This mechanism of action reduces the accumulation of oxalate in the body, limiting the burden on the kidney to filter and then excrete it in the urine. The drug is a first-in-class oral enzyme therapy in late-stage clinical development for the treatment of hyperoxaluria. The drug is being developed in Phase II for primary hyperoxaluria treatment by Allena Pharmaceutical.
Biocodex’s Stiripentol is an anticonvulsant drug used in treating epilepsy as an adjunct therapy along with Clobazam and Valproic acid. This drug is currently approved in the US, Canada, and European countries as oral tablets marketed as Diacomit for the treatment of seizures associated with Dravet syndrome in patients 2 years of age and older. In June 2020, orphan designation number EU/3/20/2290 was granted by the European Commission to Biocodex for stiripentol to treat primary hyperoxaluria, and in February 2021, Stiripentol was granted an Orphan Drug designation by the US FDA for primary hyperoxaluria treatment.
The anticipated launch of these therapies will not only give tough competition to Novo’s Rivfloza and Alnylam’s Oxlumo but also increase the primary hyperoxaluria treatment market size in the coming years. As per DelveInsight analysis, the primary hyperoxaluria market size in the 7MM is estimated to be USD 81 million in 2021, which is further expected to increase by 2032. It will be interesting to watch how Rivfloza and Oxlumo will retain their positions in the evolving primary hyperoxaluria treatment space.

Downloads
Article in PDF
Recent Articles
- Novo Nordisk to Acquire Ocedurenone; FDA Awards Orphan Drug Designation to SLS009 in AML; FDA App...
- Notizia
- Peloton raises $150M; BiomX raises $32M; Abbott and Novo collaborate; Blaze Bioscience nets $5M
- Albireo Pharma’s PFIC Therapy; Lupin’s Trichomoniasis Oral Drug; Moderna’s Weapon Aga...
- Adapsyn signed; Evolus filed; BioNTech signed a deal; Nordisk’s deal



