7 Late-Stage SLE Drugs Expected to Enter Therapeutic Domain
Dec 23, 2024
Table of Contents
Systemic lupus erythematosus (SLE) is a chronic autoimmune condition that can affect multiple parts of the body. In 2023, it was estimated that around 500,000 individuals in the United States were living with SLE, as per DelveInsight analysis. The prevalence is significantly higher in females, who account for over 90% of all cases. Among treated SLE patients in 2023, approximately 40% experienced relapse or became refractory to first-line therapy. Currently, the biologics approved by the US FDA as adjunct SLE drugs for patients are BENLYSTA and SAPHNELO. In contrast, the use of rituximab for treating SLE is considered off-label.
7 Emerging SLE Drugs in Late-stage Development
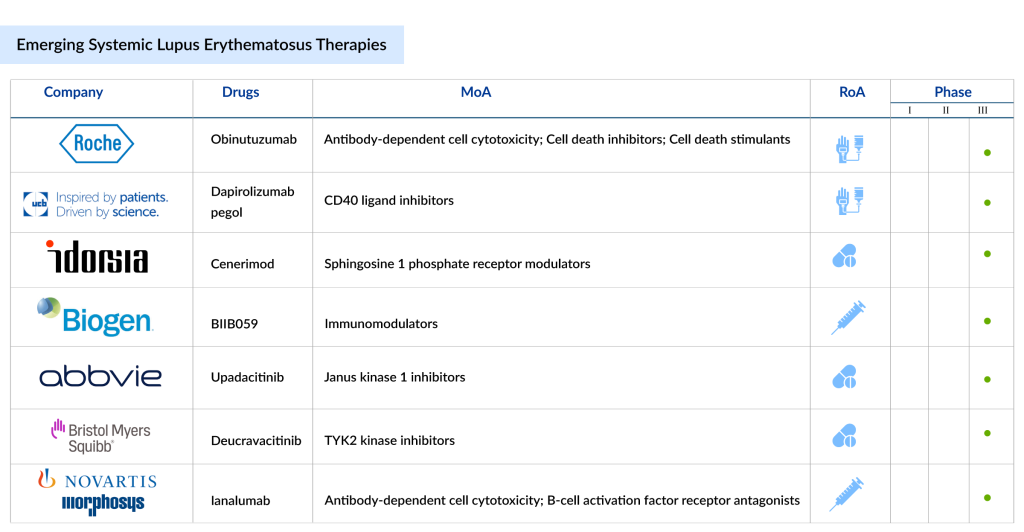
SLE pipeline possesses some drugs in late-stage developments to be approved in the near future. The emerging SLE treatment landscape holds a diverse range of therapeutic alternatives, including Litifilimab (Biogen), Ianalumab (Novartis), Cenerimod (Idorsia/Viatris), Dapirolizumab pegol (UCB/Biogen), Obinutuzumab (Roche), RINVOQ (AbbVie), Deucravacitinib (Bristol-Myers Squibb), and others in different lines of treatment. The expected launch of these therapies shall further create a positive impact on the market.
Downloads
Article in PDF
Recent Articles
- Systemic Lupus Erythematosus: An autoimmune disease
- Pfizer & Lilly’s JAK Inhibitors Drug; FDA Approves Lilly’s Bebtelovimab; GSKR...
- Is Hydroxychloroquine the answer to all the COVID-19 questions?
- Systemic Lupus Erythematosus (SLE) – Market Is Expected to Grow with Upcoming Therapies
- Aurinia’s Lupkynis for Lupus; FDA Fast Track Designation for Toripalimab; Wren Therapeutics...
Let’s take a closer look at these emerging SLE drugs.
Biogen’s Litifilimab
Litifilimab (BIIB059) is a humanized IgG1 monoclonal antibody developed by Biogen that targets blood dendritic cell antigen 2 (BDCA2). It is being studied for its potential to treat systemic lupus erythematosus and cutaneous lupus erythematosus (CLE), both chronic autoimmune diseases. This antibody binds specifically to BDCA2, a receptor found exclusively on plasmacytoid dendritic cells (pDCs), and blocks the production of type-I interferon (IFN-I) and other pro-inflammatory cytokines. This action is significant because pDCs are a major source of IFN-I in response to immune triggers, contributing to the inflammation observed in lupus. Currently, BIIB059 is in Phase III clinical trials for SLE treatment.
Novartis/MorphoSys’ Ianalumab
Ianalumab, a Novartis-developed antibody, targets the B-cell activating factor receptor (BAFF-R) to inhibit BAFF-R-mediated signaling and eliminate B cells via antibody-dependent cellular cytotoxicity. It is currently undergoing Phase III clinical trials for SLE, with the company expecting to submit it for approval by 2027, as highlighted in a recent presentation.
Idorsia Pharmaceuticals/Viatris’s Cenerimod
Cenerimod is a highly selective modulator of the sphingosine-1-phosphate receptor 1 (S1P1), administered as a once-daily oral tablet. Although the exact cause of SLE remains unclear, T and B lymphocytes are believed to be key immune cells involved in its development, with S1P1 receptors present on their surfaces. In February 2024, Idorsia Pharmaceuticals entered into a major global research and development partnership with Viatris for the advancement and commercialization of two Phase III candidates, selatogrel and cenerimod.
UCB/Biogen’s Dapirolizumab pegol
Dapirolizumab pegol is an innovative investigational treatment made up of a humanized, Fc-free antigen-binding (Fab’) fragment attached to polyethylene glycol (PEG). It functions by inhibiting CD40L signaling, which has been shown to reduce B cell activation, limit autoantibody production, decrease type 1 interferon (IFN) secretion, and suppress the activation of T cells and antigen-presenting cells (APCs).
In September 2024, UCB and Biogen announced that the Phase III PHOENYCS GO trial met its primary objective, demonstrating clinical improvements in patients with moderate-to-severe systemic lupus erythematosus. Additionally, key secondary endpoints, evaluating disease activity and flare-ups, also showed clinical benefits.
Following the positive results from the PHOENYCS GO study, UCB and Biogen are initiating a second Phase III trial of dapirolizumab pegol in 2024, named PHOENYCS FLY. Participants from the PHOENYCS GO study will continue to be monitored in a long-term open-label extension.
In November 2024, UCB and Biogen Inc. revealed comprehensive findings from the Phase III PHOENYCS GO study assessing dapirolizumab pegol. These results were presented during an oral, late-breaking session at ACR Convergence 2024, the American College of Rheumatology’s annual meeting in Washington, DC.
AbbVie’s RINVOQ
As the patent of AbbVie’s megablockbuster HUMIRA expired last year, the company has high hopes for their immunology successors RINVOQ and SKYRIZI. RINVOQ (upadacitinib) inhibits Janus kinase (JAK) enzymes within the JAK-STAT signaling pathway, which is involved in the release of pro-inflammatory cytokines. The FDA has authorized its use for treating eight autoimmune disorders: atopic dermatitis, ankylosing spondylitis, axial spondyloarthritis, Crohn’s disease, psoriatic arthritis, rheumatoid arthritis, ulcerative colitis, and polyarticular juvenile idiopathic arthritis.
In June 2024, AbbVie launched RINVOQ in the US as both a tablet and oral solution for pediatric patients aged two years and older with polyarticular juvenile idiopathic arthritis and psoriatic arthritis who did not respond adequately or were intolerant to one or more tumor necrosis factor blockers. This marks RINVOQ’s first pediatric indications, bringing its total approved indications for immune-mediated inflammatory diseases to eight. Currently RINVOQ is being evaluated in the phase III trials for SLE treatment.
Bristol-Myers Squibb’s Deucravacitinib
SOTYKTU (deucravacitinib) is an oral, selective allosteric inhibitor of tyrosine kinase 2 (TYK2) with a distinct mechanism of action, representing a new class of small molecules. It is the first selective TYK2 inhibitor being tested in clinical trials for various immune-mediated diseases. Developed by Bristol Myers Squibb, Sotyktu targets TYK2 to block the signaling of interleukin (IL)-23, IL-12, and Type 1 interferons (IFN), which are key cytokines involved in the development of several immune-mediated diseases. By binding to the regulatory domain of TYK2, Sotyktu achieves high selectivity, leading to allosteric inhibition of TYK2 and its downstream effects. At therapeutic doses, it specifically inhibits TYK2 without affecting JAK1, JAK2, or JAK3. Currently, BMS is evaluating SOTYKTU in the late stages for SLE treatment.
Roche’s Obinutuzumab
GAZYVA/GAZYVARO (obinutuzumab, RG7159, GA101) is the first glycoengineered, type II, humanized monoclonal antibody targeting CD20. It operates differently from other anti-CD20 antibodies, such as MabThera/Rituxan, and was specifically designed to target the CD20 protein on B cells with high affinity, binding to the cell surface in a type II configuration. GAZYVA has received approval in 100 countries for the treatment of various lymphoma types. In the United States, it is involved in a partnership between Genentech and Biogen. Currently, it is being evaluated in the phase III trials for SLE treatment.
SLE Treatment Space: What Lies Ahead?
The treatment landscape for systemic lupus erythematosus (SLE) is undergoing significant transformation, driven by advances in understanding its pathophysiology and the development of targeted therapies. Historically, SLE management has focused on immunosuppressive agents like corticosteroids and antimalarials to control inflammation and prevent flare-ups.
However, these therapies often come with side effects and limited efficacy in certain patient subsets. Recent breakthroughs in biologics, such as belimumab and voclosporin, have shown promising results, targeting specific immune pathways involved in SLE. Additionally, the emergence of targeted small molecules and the growing understanding of biomarkers are paving the way for personalized treatment approaches that aim to reduce disease activity while minimizing adverse effects.
DelveInsight estimates that the market size for SLE is expected to grow from USD 3.2 billion in 2023 with a significant CAGR by 2034. This growth is mainly driven by ongoing clinical research and better diagnostic tools that might improve the prognosis of the disease.
Looking ahead, the future of SLE treatment lies in the refinement of combination therapies, better patient stratification, and advancements in precision medicine. Researchers are focusing on developing therapies that not only target B cells and T cells but also modulate the complement system, cytokine pathways, and other key immune responses implicated in disease progression.
The goal is to achieve sustained remission and improve long-term outcomes for SLE patients, especially for those with refractory disease. With ongoing clinical trials and the rapid expansion of new drug classes, there is optimism that the next decade will bring more effective and individualized treatment options, offering better disease control and an improved quality of life for those affected by this complex autoimmune disorder.

Downloads
Article in PDF
Recent Articles
- Pfizer & Lilly’s JAK Inhibitors Drug; FDA Approves Lilly’s Bebtelovimab; GSKR...
- Aurinia’s Lupkynis for Lupus; FDA Fast Track Designation for Toripalimab; Wren Therapeutics...
- Aelis Farma pockets $30M; Alcyone unveils $23M for AAV gene therapies; Progentec and GSK collabor...
- Systemic Lupus Erythematosus Treatment Market: How the Leading Companies are Countering the Risin...
- Systemic Lupus Erythematosus: An autoimmune disease