Prostate Cancer Awareness Month: Early Detection Saves Lives!!
Sep 25, 2024
Table of Contents
Every September, the spotlight turns to prostate cancer. Prostate cancer stands as one of the most prevalent cancers among men, affecting approximately 1 in 8 men in the United States during their lifetime. With nearly 288,000 new cases diagnosed each year, men must prioritize regular screenings and early detection. The survival rates for prostate cancer are promising; the five-year survival rate for prostate cancer exceeds 98% when caught early, underscoring the importance of awareness and proactive health measures. Let’s delve into prostate cancer in more detail.
What Is Prostate Cancer?
Prostate cancer is a type of cancer that occurs in the prostate, a small gland located below the bladder in men. This gland produces seminal fluid, which nourishes and transports sperm. Prostate cancer typically develops when cells in the prostate gland begin to grow uncontrollably. This uncontrolled growth can form a tumor, which may remain confined to the prostate or spread to other parts of the body, such as the lymph nodes or bones.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Prostate Cancer: Metastases leading to increased incidence in the USA
- 12 Breakthrough Prostate Cancer Drugs in Late-Stage Development
- Accutar’s Phase I clinical trial for AC0176; Bio-Thera’s cancer drug, BAT6005; Nykode...
- Another Feather in the Cap for Xtandi and Keytruda — The Two Main Cancer Drugs
- VARON’s New VP Series Portable Oxygen Concentrator; Biostrap’s Wrist-Worn Digital Health Monitori...
As per DelveInsight’s estimates, the total prevalent population of prostate cancer in the 7MM was nearly 8.2 million cases in 2023, however, this figure does not reflect the treatable population. These cases are projected to increase during the forecast period, i.e., 2024–2034. The United States had the highest number of prostate cancer cases in 2023.
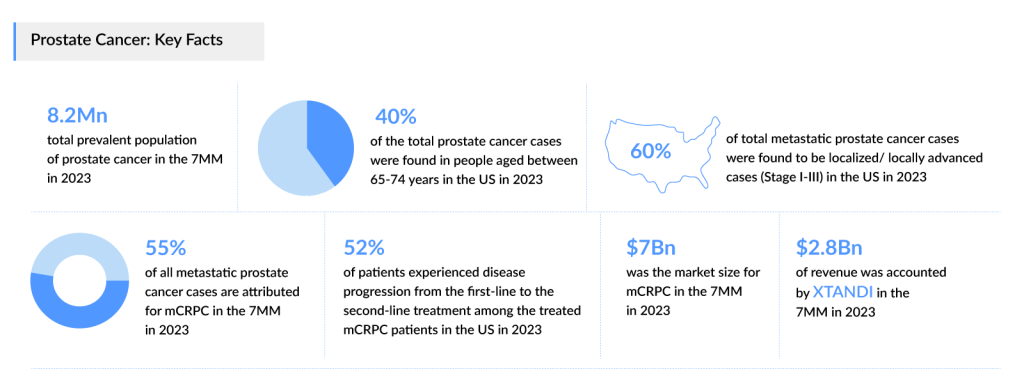
Based on DelveInsight’s assessment in 2023, the 7MM had approximately 240,300 prevalent cases of metastatic prostate cancer. These are expected to rise due to the growing geriatric population and advancements in diagnostic capabilities during the forecast period (2024−2034). Around 45% of all metastatic prostate cancer cases are attributed for mCSPC and ~55% accounted for mCRPC.
Keen to know more about metastatic prostate cancer? Get all the insights at Metastatic Prostate Cancer Market Report
Prostate cancer is most frequently diagnosed among men aged 65–74 years. Analysis indicates that age-specific data differs across various geographic regions. In the US, prostate cancer was least prevalent among individuals aged over 84 years, whereas in Japan, the lowest number of cases were observed in the age group of 54 years or younger.
Further, DelveInsight’s analysis revealed that in 2023, the US accounted for 1.5 million diagnosed prevalent cases of prostate cancer. These are expected to increase by 2034 owing to increasing aging populations, improved screening methods leading to earlier detection, changes in lifestyle and environmental factors, and advancements in medical technology allowing for better diagnosis and treatment.
Prostate Cancer Types and Stages
Prostate cancer is primarily categorized into two types: adenocarcinoma, which accounts for nearly all cases, and small cell carcinoma, a rare and aggressive form. Adenocarcinoma originates in the glandular cells of the prostate and can vary significantly in behavior. This cancer can be further classified based on its aggressiveness, typically assessed through the Gleason score, which evaluates the pattern of cancer cells under a microscope. Other, less common types include transitional cell carcinoma and neuroendocrine prostate cancer, both of which have different characteristics and treatment approaches.
The stages of prostate cancer are determined using the TNM system, which evaluates the size of the tumor (T), whether the cancer has spread to lymph nodes (N), and whether it has metastasized to distant organs (M). Staging ranges from Stage I, where the cancer is small and localized, to Stage IV, indicating advanced cancer that has spread extensively. Understanding the stage of prostate cancer is crucial for guiding treatment decisions and predicting outcomes. In addition to the TNM classification, the PSA level (prostate-specific antigen) is often used to help assess the cancer’s status and response to treatment, contributing further to a comprehensive understanding of the disease’s progression.
Understanding Prostate Cancer Symptoms
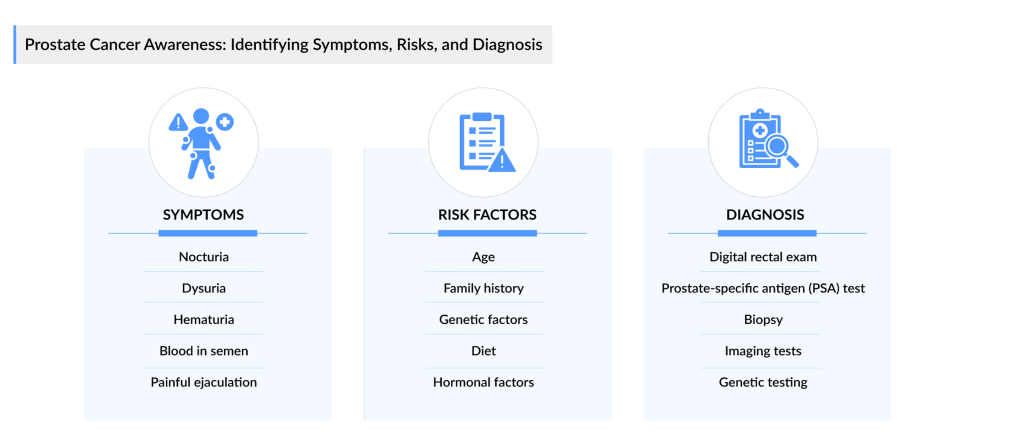
Prostate cancer often develops slowly and may not present noticeable symptoms in its early stages. However, as the disease progresses, individuals may experience a variety of signs. Common prostate cancer symptoms include difficulty urinating, such as a weak or interrupted flow, frequent urination, especially at night, and a sense of urgency. Some may also experience pain or a burning sensation during urination. Additionally, prostate cancer can lead to blood in the urine or semen and painful ejaculation. In advanced stages, it may cause lower back pain, hip pain, or discomfort in the pelvic area, often due to the spread of cancer to nearby tissues or bones. Early prostate cancer detection and awareness of these prostate cancer symptoms are crucial for effective management and treatment.
Who is at Risk of Prostate Cancer?
Prostate cancer risk factors are varied and can be categorized into non-modifiable and modifiable factors. Non-modifiable risk factors include age, race, and family history. The likelihood of developing prostate cancer increases significantly with age, particularly after the age of 50. Additionally, men of African descent are at a higher risk compared to their Caucasian and Asian counterparts. A family history of prostate cancer also raises an individual’s risk, suggesting a genetic predisposition that can contribute to the development of the disease.
Modifiable prostate cancer risk factors, on the other hand, are lifestyle-related and can be influenced by personal choices. Research indicates that obesity is linked to an increased risk of aggressive prostate cancer. Diet plays a critical role as well; a high intake of red meat and high-fat dairy products may contribute to the risk, while a diet rich in fruits and vegetables may have a protective effect. Moreover, physical activity and maintaining a healthy weight are associated with a lower risk of prostate cancer. Understanding these risk factors is essential for men to take preventative measures and make informed health choices.
The Importance of Early Detection and Awareness of Prostate Cancer
Early detection of prostate cancer significantly enhances the likelihood of successful treatment and recovery. When identified in its early stages, prostate cancer is often manageable and can be treated effectively, leading to better survival rates. Regular prostate cancer screenings allow for the detection of the disease before prostate cancer symptoms appear, providing an opportunity for intervention at a stage when treatment options are more varied and less invasive. Moreover, advancements in medical technology and treatment modalities have made it possible to tailor therapies to individual patients, further improving outcomes.
Additionally, awareness also plays a pivotal role in encouraging men to recognize the risk factors and symptoms associated with prostate cancer. Many men are unaware that they are at risk, particularly those over the age of 50, or those with a family history of the disease. By educating men about the importance of regular prostate cancer screenings, such as prostate-specific antigen (PSA) tests and digital rectal exams (DRE), healthcare providers can help facilitate earlier prostate cancer diagnosis and treatment. Increased awareness can also help dispel myths and stigmas surrounding prostate cancer, encouraging men to seek medical advice and support.
Thus, promoting awareness and fostering a proactive approach to screening can save lives and improve the quality of life for those diagnosed with prostate cancer.
Advances in Prostate Cancer Treatment and Research
Prostate cancer treatment has evolved significantly in recent years, offering patients a range of options tailored to the stage and aggressiveness of the disease. The primary treatment modalities include surgery, radiation therapy, and active surveillance. For localized prostate cancer, radical prostatectomy or radiation therapy is often recommended, aiming to remove or destroy cancer cells while preserving surrounding tissue.
Hormone therapy may sometimes be utilized as well. Patients who have never undergone androgen deprivation therapy (ADT) and are responsive to it are referred to as having hormone-sensitive prostate cancer (HSPC) or castrate-sensitive prostate cancer (CSPC). The primary treatments for non-metastatic castration-resistant prostate cancer (nmCRPC) include ADT, also known as hormone therapy, second-line ADT, and active surveillance. If the cancer has spread to the bones or other areas beyond the prostate, treatment options may include pain relief medications, bisphosphonates, RANK ligand inhibitors, hormonal therapy, chemotherapy, radiopharmaceuticals, immunotherapy, focused radiation, and various targeted therapies.
Approved Drugs for Prostate Cancer Treatment
Current therapies for metastatic prostate cancer treatment include Astella/Pfizer’s XTANDI and Janssen’s ZYTIGA, both of which have been on the market for over a decade. Even though ZYTIGA’s generics have entered the US market since 2019 and the EU since late 2022, leading to a drastic decline in the revenue mainly in the US, the product is extensively being evaluated in combination with novel emerging therapies, leading to an increase in patient share on the compound abiraterone acetate.
Janssen expanded its portfolio with the introduction of ERLEADA in 2019 for mHSPC, following its 2018 approval for nmCRPC. Additionally, Bayer’s NUBEQA has quickly gained traction and is emerging as a strong competitor.
Moreover, PARP inhibitors are making their mark in patients with HRR gene mutations (BRCA1/2). AstraZeneca’s LYNPARZA was introduced for 1L+ prostate cancer treatment, while Pharma& Schwiez’s RUBRACA became available for 3L mCRPC patients in 2020. Notably, in 2023, Pfizer’s TALZENNA, Janssen Pharmaceuticals’ AKEEGA, and AstraZeneca/Merck Sharp & Dohme’s LYNPARZA were also approved for use in the first-line setting. Drugs such as Myovant Sciences’ ORGOVYX and Sanofi’s JEVTANA have also received regulatory approval for mCRPC in the United States.
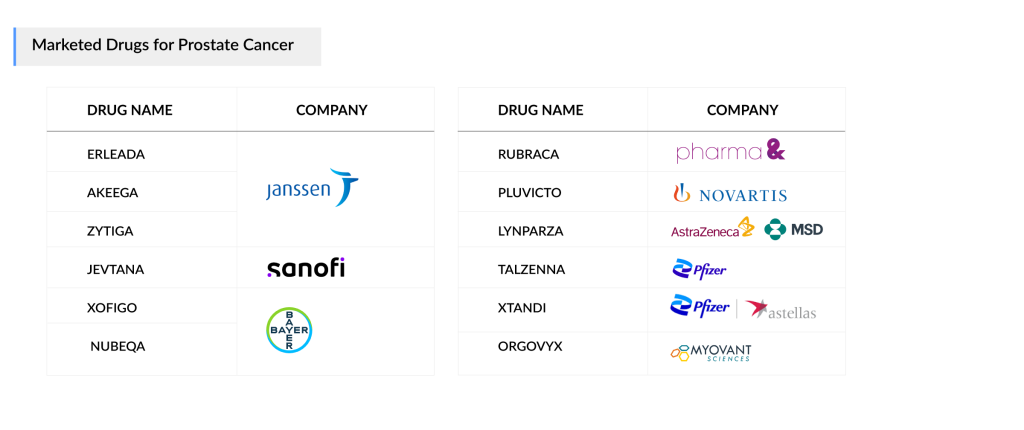
The mCRPC treatment market saw the approval of Novartis’ radioligand therapy, PLUVICTO, in 2022, leading to unexpectedly high revenue in the third-line mCRPC segment. Novartis aims to further extend its reach into earlier-line mCRPC by 2024 and mHSPC by 2025 in the US market.
Explore the breakthroughs in mCRPC treatment – discover why it’s leading prostate cancer research
Emerging Therapies for Prostate Cancer Treatment on the Horizon
The prostate cancer pipeline possesses some drugs in mid and late-stage developments to be approved in the near future. Quantitatively mCRPC pipeline seems to be quite strong, however with recent failures/terminations of several trials of certain therapies such as KEYTRUDA and OPDIVO in the past year.
As per analysis, some of the potential prostate cancer drugs include ESSA Pharma’s EPI-7386, Macrogenics’s and Daiichi Sankyo anti-B7-H3 antibody-drug conjugates, MGC-018 and DS-7300, respectively, Arvinas’s AR-targeted PROTAC protein degrader (ARV-766), Point Biopharma’s radioligand therapy (177Lu-PNT2002), Exelixis’s multiple receptor tyrosine kinase (Cabozantinib), and Promontory Therapeutics’s pyrophosphate conjugate (PT-112).
Some of the other prostate cancer drugs in the late stage of development include 177Lu-PSMA-I&T (Curium), Opevesostat (MK-5684) (Merck and Orion), 177Lu-DOTA-rosopatamab (Telix Pharmaceuticals), Capivasertib (AstraZeneca), Masitinib (AB Science), FPI-2265 (Fusion Pharma), Mevrometostat (PF-06821497) (Pfizer), Fuzuloparib (Jiangsu Hengrui Pharmaceuticals), ModraDoc006 (Modra Pharmaceuticals), BMS-986218 (Bristol-Myers Squibb), Lorigerlimab (MacroGenics), SX-682 (Syntrix Pharmaceuticals), ZEN-3694 (Zenith Epigenetics), Vudalimab (Xencor), Zenocutuzumab (Merus), Vobramitamab Duocarmazine (MacroGenics), LAE201 (Laekna Therapeutics), TAVT-45 (Tavanta Therapeutics), pTVG-HP (MVI-816) (Madison Vaccines), TAS-115 (Taiho Pharmaceutical), KPG-121 (Kangpu Biopharmaceuticals), and CAN-2409 (Candel Therapeutics).
On the other hand, the emerging landscape of mCSPC treatment also holds a diverse range of therapeutic alternatives for treatment, including PLUVICTO (Novartis), TALZENNA + XTANDI (Pfizer), Saruparib (AstraZeneca), TRUQAP + abiraterone (AstraZeneca), AKEEGA (Niraparib/abiraterone acetate) + prednisone (Janssen), NUBEQA + ADT (Bayer), ORGOVYX (Myovant Sciences/Sumitomo Pharma), and others. In addition to these, Jiangsu Hengrui is evaluating dalpiciclib + abiraterone + prednisone and SHR3680 for treating mCSPC, in separate prostate cancer clinical trials, in China.

The expected launch of these prostate cancer therapies shall further create a positive impact on the market.
Discover more about mCSPC drugs in development at Metastatic Castration-Sensitive Prostate Cancer Clinical Trials
Prostate Cancer Market Dynamics and Trends
The prostate cancer therapeutic landscape is marked by intense competition, with numerous approved treatments currently available in the market and several promising therapies in the pipeline addressing unmet needs in both conditions.
As per DelveInsight analysis, the total market size of mCRPC in the 7MM in 2023 was approximately USD 7 billion. On the other hand, the total market size of mCSPC in the 7MM in 2023 was USD 2.6 billion. This is anticipated to grow by 2034 at a moderate CAGR.
As per the estimates, the US accounted for the largest market size of mCRPC and mCSPC in 2023, i.e., nearly 66% in comparison to EU4 (Germany, Italy, France, and Spain) and the UK, and Japan. In the current market, XTANDI accounted for the major share, which was around USD 2.8 billion in 2023. However, XTANDI sales are expected to decline due to the US Inflation Reduction Act (IRA) impact which is scheduled to be effective Jan 2025.
The prostate cancer market is expected to witness significant growth owing to the rising prevalence of prostate cancer cases, the emergence of novel, more accurate, next-generation imaging techniques, extensive market penetration of approved therapies in mCRPC due to label expansions, and the entry of new emerging therapies.
However, challenges such as high treatment costs, regulatory hurdles, and disparities in healthcare access may impact market expansion. Overall, the interplay between these factors is creating a dynamic landscape in the prostate cancer market, offering opportunities for growth while also necessitating strategies to address existing barriers.
Key Highlights from the ESMO 2024 and ASCO 2024 Conferences
The ASCO and ESMO conferences serve as pivotal platforms for presenting the latest advancements in oncology research including prostate cancer. At these events, researchers and clinicians share groundbreaking data on novel therapies, clinical trial results, and innovative treatment strategies, shaping the future of prostate cancer management.
ESMO 2024 Highlights
The majority of abstracts focus on mCRPC with BMS’ dual androgen receptor (AR) ligand-directed degrader and antagonist in heavily pretreated mCRPC, Bayer/Astellas’ Phase III trial comparing XTANDI vs XTANDI + XOFIGO in asymptomatic or mildly symptomatic mCRPC, Exelixis/Ipsen’s overall survival data of Cabozantinib and Atezolizumab combo from pivotal CONTACT-02 trial in mCRPC, whereas, Amgen and Epizyme are coming up with novel classes, a STEAP1 x CD3 XmAb 2+1 Immune Therapy and EZH2 inhibitor respectively in early stages.
Below are the potential prostate cancer data readouts during the ESMO 2024 conference:
Get an exclusive ESMO 2024 Conference analysis of all the abstracts presented during the conference
ASCO 2024 Highlights
Below are the potential prostate cancer data readouts held at the ASCO 2024 conference:
Get an exclusive ASCO 2024 Conference analysis of all the abstracts presented during the conference
Final Thoughts on Prostate Cancer
Prostate cancer is a significant health concern that affects millions of men and their families, but it doesn’t have to define their lives. Awareness and education are our most powerful tools in this battle. Each year, Prostate Cancer Awareness Month serves as a critical reminder that understanding the disease and taking proactive steps can lead to better outcomes.
As we recognize Prostate Cancer Awareness Month, let’s commit to taking action. Advocate for regular screenings, support those affected, and continue to educate ourselves and our communities. Together, we can build a future where prostate cancer is detected early, treated effectively, and eventually, significantly reduced.
This September, let’s stand united in the fight against prostate cancer. It’s time to turn awareness into action—because every conversation, every screening, and every shared story can make a difference. Don’t wait—reach out, educate yourself, and encourage the men in your life to do the same. Early detection is not just a slogan; it’s a lifeline.

Downloads
Article in PDF
Recent Articles
- Illuccix Receives Dutch Approval for PSMA-PET Imaging in Prostate Cancer; FDA Approves Valcare Me...
- Key Updates on Phase 1 Trial of AB-1005 Gene Therapy for Multiple System Atrophy-Parkinsonian Typ...
- J&J’s 2-in-1 Tablet for Prostate Cancer; FDA Approves TALVEY for Heavily Pretreated Multiple ...
- FDA Approves AstraZeneca’s Enhertu; Bayer Wins FDA Approval for Prostate Cancer Therapy, Nubeqa; ...
- Incyte’s MONJUVI Combo Approved by FDA for Relapsed/Refractory Follicular Lymphoma; Gilead’s YEZT...



