Madrigal’s Rezdiffra Breakthrough Transforms NASH Treatment, Leading the Way for Next-Gen Therapies
Sep 04, 2024
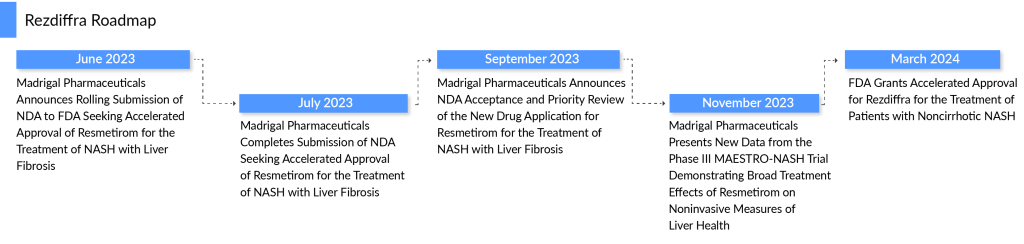
The prolonged wait for an effective remedy for non-alcoholic steatohepatitis (NASH) has concluded with the FDA’s approval of the inaugural drug for this fatty liver condition. Madrigal Pharmaceuticals’ resmetirom, soon available as Rezdiffra, has achieved the milestone of becoming the primary therapy for NASH to receive FDA clearance, after a protracted journey through drug development.
On March 14, 2024, the FDA greenlit this tablet medication for treating NASH, in cases of moderate to severe liver scarring, or fibrosis, at stages F2 and F3. The anticipated acceptance of Rezdiffra is poised to pave the way for forthcoming NASH treatments, establishing a standard for effectiveness and safety among upcoming contenders.
NASH is the progressive form of liver injury that carries a risk of progressive fibrosis, cirrhosis, and end-stage liver disease. In 2023, prominent liver disease organizations, including the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL), officially redefined Nonalcoholic Steatohepatitis (NASH) to Metabolic Dysfunction-Associated Steatohepatitis (MASH), aligning the name more closely with its metabolic roots. For the most up-to-date insights, explore our comprehensive MASH Market Report.
Downloads
Article in PDF
Recent Articles
- Notizia
- Tepezza receives approval; a new way to treat Alzheimer’s
- Beyond Supportive Care: How New Drugs are Shaping Metabolic-associated Steatohepatitis (MASH) Tre...
- Intellia’s Quest with CRISPR; Innovent, Synaffix ADC Tech Deal; Lilly’s Diabetes Bloc...
- Redefining Liver Disease in the Metabolic Era: From NASH to MASH and the Rise of Obesity‐targeted...
Resmetirom, the primary component of Rezdiffra, is a compact molecule designed to target the liver. It operates by stimulating the thyroid hormone receptor beta (THR-β), a receptor abundant in the liver. Activating THR-β through this drug aids in decreasing levels of triglycerides within the liver and enhancing the metabolism of lipids.
Madrigal has set the wholesale acquisition price for Rezdiffra at $47,400, without factoring in any discounts. Previously, the influential group at the Institute for Clinical and Economic Review estimated that the drug would be considered cost-effective by common standards if its final price ranged between $39,600 and $50,100 per year.
In a significant victory for Madrigal, the FDA has decided not to mandate a liver biopsy to determine a patient’s eligibility for Rezdiffra. Before this approval, the potential requirement for a biopsy was a major topic of debate among investors, as it was seen as a barrier limiting access to Rezdiffra due to its cumbersome and invasive nature.
Bill Sibold, Madrigal’s CEO, expressed that the approval of Rezdiffra marks a significant milestone in the treatment of NASH, particularly for patients with moderate to advanced liver fibrosis. This approval, achieved after over 15 years of dedicated research led by Dr. Becky Taub and a small R&D team, addresses a critical gap in available therapies. Sibold highlighted the historic nature of this moment for the NASH field, showcasing the remarkable capabilities of the pharmaceutical industry. He emphasized the company’s enthusiasm for providing Rezdiffra to those who require it most.
Becky Taub, M.D., the Founder, Chief Medical Officer, and President of Research & Development at Madrigal, expressed gratitude to the numerous patients whose participation in our clinical trials made the accelerated approval of Rezdiffra achievable. It is our belief at Madrigal that Rezdiffra will revolutionize the approach to treating NASH in patients with moderate to advanced liver fibrosis. This liver-directed therapy is anticipated to assist physicians in enhancing fibrosis and resolving NASH, thereby preventing the progression of cirrhosis in their patients.
Rezdiffra marked a significant milestone as the first medication to achieve both NASH resolution and improvement in fibrosis during a phase III trial. Before Rezdiffra’s breakthrough, Intercept’s Ocaliva seemed poised to be the first in this space until the FDA rejected it for the second time in June 2023. Many other pharmaceutical giants such as Pfizer, Bristol Myers Squibb, Genfit, NGM Biopharmaceuticals, among others, have faced setbacks in the expansive field of NASH drug development.
The recent approval of Rezdiffra was based on an analysis of approximately 900 patients diagnosed with NASH and liver fibrosis through biopsies, who participated in the MAESTRO-NASH trial. Results from the study showed that Rezdiffra, administered at either 80mg or 100mg once daily, led to NASH clearance without worsening fibrosis in 25.9% and 29.9% of patients respectively, compared to 9.7% among those who received a placebo. These findings were published in the New England Journal of Medicine.
Furthermore, 24.2% and 25.9% of patients receiving the two different doses of Rezdiffra experienced an advancement in fibrosis by at least one stage, without any worsening in the fatty liver disease activity score. These rates were notably higher compared to the 14.2% observed in the placebo group.
In the official label approved by the FDA, Rezdiffra’s effectiveness for each specific outcome was evaluated through assessments by two pathologists. When it came to enhancing liver fibrosis and preventing the deterioration of steatohepatitis, pathologist A noted a 13% difference (adjusted for placebo) with Rezdiffra at 100mg, while pathologist B reported an 11% rate.
According to the FDA’s approval, patients will be prescribed varying doses of Rezdiffra depending on their body weight. Those weighing less than 100kg (220lbs.) are advised to take 80mg, while others are recommended the 100mg strength.
Madrigal also shared additional safety data from the MAESTRO-NAFLD-1 trial, which the company believes mimics real-world scenarios where patients’ conditions are not confirmed through biopsies but rather through noninvasive measures. In this trial, the drug was well tolerated and exhibited significant enhancements in certain biomarkers such as liver fat and triglyceride levels.
While Rezdiffra benefits from being the first to enter the NASH treatment market, other treatments have shown encouraging early clinical results, aiming to capture a significant portion of the NASH treatment market.
According to DelveInsight’s evaluation in 2022, there were an estimated 21 million prevalent cases of NASH in the US. Out of these, a total of 9 million cases were diagnosed, and this number is projected to increase by the end of 2032 in the US. As the prevalence of NASH continues to rise, researchers and pharmaceutical companies are actively exploring innovative therapeutic approaches.
Viking Therapeutics’ VK2809, an oral thyroid receptor-beta agonist similar to Rezdiffra. In a midstage trial, VK2809 resulted in an average 51.7% decrease in liver fat after 12 weeks of treatment. However, the company has not yet released data for the 52-week histologic response.
On the other hand, 89bio’s pegozafermin and Akero Therapeutics’ efruxifermin are both FGF21 analogs administered subcutaneously. They have demonstrated promising outcomes in reducing fibrosis in patients with F2 and F3 NASH. Phase III trials for these drugs are either underway or pending.
Just before the approval of Rezdiffra, Ionis announced that a high dose of its antisense drug ION224 helped 32% of patients achieve a one-stage improvement in liver fibrosis without worsening steatohepatitis by week 51 in a phase II trial, compared to 12.5% in the placebo group.
Next, we have the GLP-1 agonist group. In February, Lilly shared encouraging results from the phase II NASH trial of its GIP/GLP-1 dual agonist tirzepatide, designed for overweight or obese individuals. However, it’s important to note that the study wasn’t statistically designed to assess improvements in fibrosis. This medication is already approved for diabetes under the name Mounjaro and for obesity as Zepbound.
Madrigal, as part of the accelerated approval process, has agreed to furnish additional evidence validating the benefits of Rezdiffra. This strategy involves conducting the ongoing 54-month extension of the MAESTRO-NASH study, which will assess disease progression along with outcomes such as liver transplants and the onset of cirrhosis. Furthermore, a separate investigation named MAESTRO-NASH Outcomes is examining Rezdiffra’s effects on patients with well-compensated NASH cirrhosis, a more advanced stage of the illness.
If the results are favorable, this research could facilitate the complete approval of Rezdiffra and broaden its patient population. Sibold noted that pinpointing an exact timeline for results is challenging due to the event-driven nature of the trial, but he approximated an outcome around 2026 or 2027.
While MAESTRO-NASH is monitored for 54 months in its long-term follow-up, Sibold mentioned that Rezdiffra is anticipated to serve as an ongoing therapy, administered until the patient’s condition subsides, including the elimination of underlying factors like obesity.
In May 2024, Madrigal Pharmaceuticals reported promising initial uptake for REZDIFFRA during its Q1 earnings call, highlighting “tremendous interest” in the therapy, particularly among specialists treating NASH. CEO Bill Sibold noted that the company is targeting around 14,000 specialists who manage approximately 315,000 eligible NASH patients, with encouraging early metrics despite being less than a month post-launch. Although Madrigal’s sales team has connected with over 80% of top targets—who have generated 75% of REZDIFFRA prescriptions—the company missed Street forecasts for Q1 2024, a quarter that didn’t yet include REZDIFFRA sales, while SG&A expenses surged nearly fivefold to $80.8 million.
In April 2024, Madrigal Pharmaceuticals, Inc. announced that REZDIFFRA is now available in the U.S. as the first FDA-approved therapy for NASH in adults with noncirrhotic NASH and moderate to advanced liver fibrosis (stages F2 to F3). Madrigal’s efforts include partnering with healthcare providers, payers, and patient advocates to ensure patients can access REZDIFFRA and achieve their treatment goals. The company’s field teams are fully deployed, the specialty pharmacy network is operational, and the first patients have already begun receiving REZDIFFRA.
In addition to offering this new treatment option, Madrigal has established the Madrigal Patient Support program to help eligible patients navigate insurance and affordability challenges, including providing co-pay support and access to medication through a patient assistance program (PAP) for those without insurance. REZDIFFRA’s prescribing information emphasizes a patient-centric approach, with no biopsy requirement for diagnosis, moving the field towards noninvasive testing options for NASH. REZDIFFRA should not be used in patients with decompensated cirrhosis and is distributed through a limited specialty pharmacy network.
The approval of REZDIFFRA as the first FDA-approved therapy for NASH marks a pivotal moment in the treatment of liver diseases. This breakthrough not only provides a new, effective option for patients with moderate to advanced liver fibrosis but also signals a significant shift in the therapeutic landscape for NASH. With REZDIFFRA’s success, the door is now open for other companies to advance their NASH treatments, spurring innovation and potentially leading to a wave of new therapies entering the market. As we move forward, this approval sets a new standard in the fight against liver diseases, offering hope for millions of patients and energizing the broader pharmaceutical industry to continue developing novel solutions.

Downloads
Article in PDF
Recent Articles
- Redefining Liver Disease in the Metabolic Era: From NASH to MASH and the Rise of Obesity‐targeted...
- Empowering Change: REZDIFFRA’s Trailblazing Journey in NASH Treatment
- From NASH to MASH: Unraveling the Evolution and Future of Liver Disease Treatment
- Albireo Pharma’s PFIC Therapy; Lupin’s Trichomoniasis Oral Drug; Moderna’s Weapon Aga...
- AZ announces positive results for durvalumab and tremelimumab; FDA nods to extend LILETTA’s use t...