Understanding Rheumatoid Arthritis: Epidemiology Trends and Market Insights
Oct 02, 2024
Table of Contents
Rheumatoid arthritis is a chronic autoimmune disease that wreaks havoc on the joints, causing progressive and destructive polyarthritis. According to the World Health Organization, approximately 18 million people globally live with this condition, with about 1.5 million cases reported in the U.S. Characterized by relentless pain and joint degradation, rheumatoid arthritis typically advances from the smaller, distal joints to larger, proximal ones. Although the exact causes of this debilitating condition remain elusive, we know that it arises from an immune response where the body’s defense system mistakenly attacks its own healthy cells. Notably, patients with rheumatoid arthritis are predominantly female, with approximately 75% of cases affecting women, and it usually manifests between the ages of 30 and 50.
How Is Rheumatoid Arthritis Diagnosed?
Diagnosing rheumatoid arthritis is a multifaceted process that combines a variety of factors. Physicians assess a patient with rheumatoid arthritis based on symptoms, medical history, and family background, alongside conducting a thorough physical examination. Advanced imaging techniques, such as ultrasound sonography, may also be utilized. Additionally, laboratory tests often reveal elevated levels of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) in serum, alongside the detection of specific autoantibodies associated with RA, forming a key part of the rheumatoid arthritis medical procedure.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Outbreak of Companies Challenging New Pathways for Treatment of Pulmonary Arterial Hypertension
- SEC scrutinizes; Acacia meets endpoint; Axovant’s dementia drug; Novartis expands; Aurobind...
- What are your views on Gene Therapy?
- Understanding the Acute Myeloid Leukemia Surge and the Road to Solutions
- Prosecutors rope Pfizer; Pharma groups to FDA; Regeneron simulates; Otsuka & Lundbeck revive...
Rheumatoid Arthritis Epidemiology
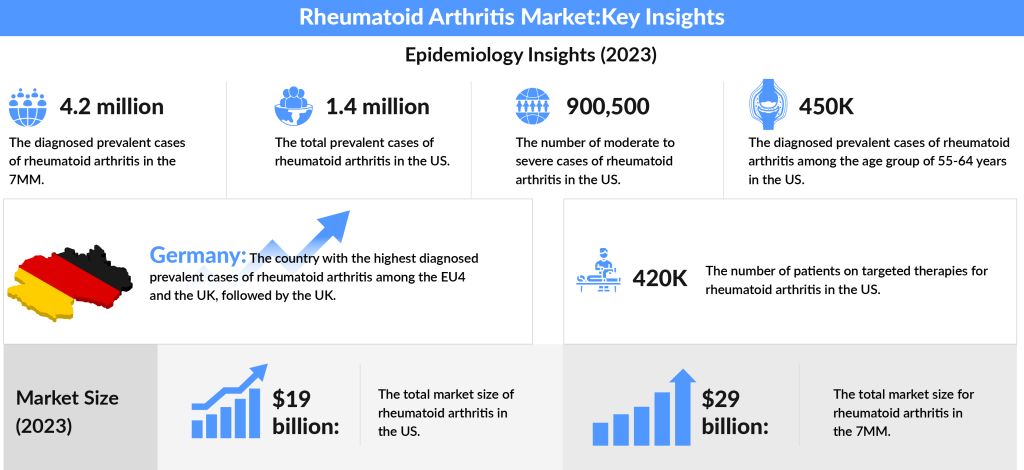
In exploring the prevalence of rheumatoid arthritis, it’s evident that the landscape is complex. The rheumatoid arthritis prevalence is staggering. In 2023, the total diagnosed prevalent cases of RA across the 7MM (the United States, Japan, EU4, and the UK) were estimated to be around 4.2 million, with expectations for this number to rise during the study period. Focusing on the U.S., approximately 1.4 million individuals were living with RA in 2023. Among the EU4 countries (Germany, France, Italy, and Spain) and the UK, Germany topped the charts with the highest number of moderate to severe RA cases, followed closely by the UK.
The landscape of therapy for rheumatoid arthritis is also evolving. In the U.S., about 420K patients were receiving targeted therapy for rheumatoid arthritis in 2023, and this figure is expected to grow in the coming years. Notably, the age group of 55-64 years represented the largest segment of rheumatoid arthritis treatment cases, accounting for around 450K, followed by those aged 65 and older. The increasing use of treatment for rheumatoid arthritis and evolving strategies underscore the importance of early diagnosis and therapy for rheumatoid arthritis to improve patient outcomes.
How do you Treat Rheumatoid Arthritis?
The battle against rheumatoid arthritis often begins with conventional rheumatoid arthritis treatment options, specifically disease-modifying anti-rheumatic drugs (DMARDs). Medications for rheumatoid arthritis like methotrexate (RHEUMATREX, TREXALL), leflunomide, hydroxychloroquine, and sulfasalazine are commonly prescribed to temper the immune system’s overactivity, alleviating pain, swelling, and stiffness while protecting joint integrity. Methotrexate serves as the cornerstone of treatment for rheumatoid arthritis, frequently used in combination therapies alongside glucocorticoids (GCs), other csDMARDs, and biologics. For patients with rheumatoid arthritis who don’t respond adequately to csDMARDs or present severe symptoms, a variety of biologics come into play, including anti-TNF agents, T-cell inhibitors, B-cell inhibitors, interleukin inhibitors, and targeted synthetic DMARDs like JAK inhibitors.
In the broader context of the rheumatoid arthritis treatment landscape, various classes of rheumatoid arthritis medicines are utilized, including non-steroidal anti-inflammatory agents (NSAIDs), corticosteroids, and DMARDs. NSAIDs are often the first line of treatment for rheumatoid arthritis due to their rapid onset of action. They alleviate pain and reduce inflammation, helping patients with rheumatoid arthritis manage discomfort. Notable medications for rheumatoid arthritis in this category include ADVIL, MOTRIN, NUPRIN (ibuprofen), ALEVE (naproxen), and prescription options like MOBIC (meloxicam), CATAFLAM, VOLTAREN, ARTHROTEC (diclofenac), and INDOCIN (indomethacin). The older NSAID, ASPIRIN, has largely fallen out of favor due to its gastrointestinal toxicity. Additionally, COX-2 inhibitors, like CELEBREX (celecoxib), were designed to reduce gastrointestinal risk but have raised concerns over cardiovascular safety, leading to the withdrawal of agents like VIOXX (rofecoxib) and BEXTRA (valdecoxib).
Corticosteroids, such as MEDROL (prednisone, methylprednisolone), possess both anti-inflammatory and immunoregulatory properties. They can be administered orally, intravenously, or through injections directly into the joints as part of a rheumatoid arthritis medical procedure. Corticosteroids serve as valuable temporary therapy for rheumatoid arthritis while waiting for DMARDs to take effect and can also act as a chronic adjunctive treatment for severe cases not well controlled by NSAIDs and DMARDs. 1st line treatment for rheumatoid arthritis often involves PREDNISONE, typically prescribed in doses ranging from 5 to 10 mg daily, though higher initial doses may be used. This is critical in understanding how rheumatoid arthritis is treated during the initial stages of disease progression.
DMARDs play a vital role in rheumatoid arthritis treatment therapy by not only alleviating symptoms but also modifying the disease’s course and improving radiographic outcomes. Unlike NSAIDs and corticosteroids, which provide more immediate relief, DMARDs may take several weeks or even months to demonstrate their full therapeutic effects. RHEUMATREX, TREXALL (methotrexate) is widely recognized as the best treatment for RA, celebrated for its efficacy and favorable toxicity profile. Other notable medications for rheumatoid arthritis include PLAQUENIL (hydroxychloroquine), AZULFIDINE (sulfasalazine), and ARAVA (leflunomide), all contributing to long-term disease management.
Within the DMARD category, Tumor Necrosis Factor (TNF) Inhibitors represent a crucial subclass. These agents target inflammatory processes more directly and include ENBREL (etanercept), HUMIRA (adalimumab), REMICADE (infliximab), CIMZIA (certolizumab pegol), and SIMPONI (golimumab). Beyond these, other significant DMARDs further enhance treatment for rheumatoid arthritis. ORENCIA (abatacept) acts as a T-cell costimulatory blocking agent, while RITUXAN (rituximab) serves as a B-cell depleting agent. Additional agents include ACTEMRA (tocilizumab), an interleukin-6 inhibitor, and KINERET (anakinra), an interleukin-1 receptor antagonist. The list also features AURANOFIN, an intramuscular gold compound, as well as immunomodulatory and cytotoxic options like IMURAN (azathioprine) and NEORAL, SANDIMMUNE (cyclosporine a).
Given the potential for significant cartilage damage and bony erosions within the first two years of rheumatoid arthritis, rheumatologists are increasingly proactive in initiating DMARD therapy as soon as a diagnosis is confirmed. While NSAIDs and corticosteroids primarily address symptom management, it is the DMARDs that play a critical role in altering the long-term trajectory of rheumatoid arthritis, ensuring better outcomes and improved quality of life for patients with rheumatoid arthritis.
Many patients with rheumatoid arthritis often wonder about how to treat RA permanently. While there is currently no definitive cure, ongoing research into alternative treatments continues to provide hope. Emerging rheumatoid arthritis treatment therapy and lifestyle modifications can complement traditional approaches, offering a more holistic view of managing this chronic condition. What treats rheumatoid arthritis and finding the safest drug are crucial steps toward achieving better health outcomes and enhancing the overall quality of life for those affected by the disease.
The prevalence of rheumatoid arthritis has also driven the rapid expansion of the rheumatoid arthritis treatment market. In 2023, the total market size in the U.S. alone was estimated at approximately USD 19 billion. Across the 7MM (the United States, Japan, and EU4, and the UK), this figure balloons to around USD 29 billion, reflecting substantial demand for effective rheumatoid arthritis treatment therapies. This market is poised for growth during the forecast period from 2024 to 2034, driven by innovations in treatment options.
Notably, Rabeximod stands out as a promising candidate, addressing critical gaps in rheumatoid arthritis management with its beneficial tolerability and unique mechanism of action, making it suitable for patients with rheumatoid arthritis in both early and late stages of the disease. However, the entry of biosimilars into the market—such as those referencing REMICADE—has already begun to exert downward pressure on sales values, a trend expected to accelerate with the anticipated launch of biosimilars and generics for blockbuster drugs like HUMIRA in 2023 and XELJANZ in 2025. This impending competition is likely to have a pronounced impact on the growth trajectory of the rheumatoid arthritis treatment market, necessitating a strategic adaptation from both pharmaceutical companies and healthcare providers.
What Does the Future Hold for the Rheumatoid Arthritis Market?
While there is currently no cure for rheumatoid arthritis and no treatment can reverse the disease’s progression, the objective remains to manage pain and inflammation effectively, aiming for remission or at least low disease activity. Unfortunately, the FDA has approved only a handful of drugs that can slow the disease’s course and improve patients’ quality of life. Consequently, RA management tends to be supportive and symptom-based.
In recent years, there has been a surge in research focused on novel treatment strategies, including monoclonal antibodies and small molecules. Approved drugs for RA treatment include OLUMIANT, RINVOQ, and ORENCIA. Historically, HUMIRA was one of the top-selling drugs globally, paving the way as the first human monoclonal antibody approved for RA. However, with its patent recently expiring, numerous companies are now launching biosimilars to fill the gap.
Despite recent advancements, there’s a critical need for deeper understanding of RA and its unmet needs. To tackle these challenges, allocating resources toward research and development (R&D) is essential. Successful pricing policies will also be key in launching attractive and relevant products in the market. Many companies are already embarking on clinical trials to explore new treatment options or improve existing therapies.
What Promising Therapies Are in the Pipeline for Rheumatoid Arthritis?
As we delve into the evolving landscape of rheumatoid arthritis, it becomes increasingly clear that innovation is at the forefront of treatment strategies. With around 18 million people affected globally—approximately 1.5 million in the U.S. alone—the quest for more effective therapies has never been more pressing. New players are entering the stage, and groundbreaking therapies are emerging, giving patients and healthcare providers alike a renewed sense of hope.
At the heart of this revolution are JAK inhibitors, which have firmly established themselves as the backbone of rheumatoid arthritis management. Among these, RINVOQ has soared to prominence, celebrated for its efficacy in quelling the chronic inflammation that characterizes rheumatoid arthritis. However, its rise has not been without caution; AbbVie recently updated RINVOQ’s labeling to reflect serious safety concerns, including risks of cancer and blood clots. This highlights a dual narrative in the realm of rheumatoid arthritis treatments: while innovation paves the way for effective management, vigilance regarding safety is paramount.
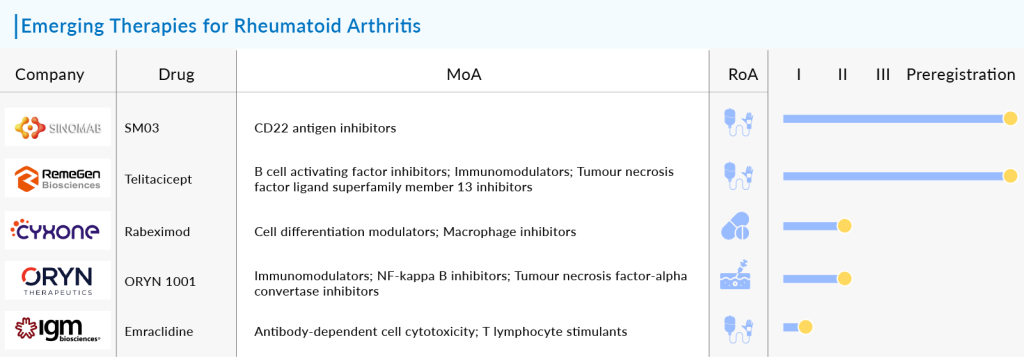
In addition to JAK inhibitors, macrophage inhibitors are emerging as a targeted approach to combat RA’s destructive inflammation. One of the standout candidates in this category is Rabeximod, developed by Cyxone. This orally administered drug operates uniquely by specifically targeting the NFκB pathway in inflammatory macrophages—the very cells that orchestrate the inflammatory response in RA. Clinical trials have shown promise, suggesting that Rabeximod may not only slow disease progression but also ease the severity of symptoms in patients. Following a successful pre-IND meeting with the FDA in June 2022, Cyxone is set to advance Rabeximod to a Phase IIb study, a critical step that could bring this innovative treatment closer to the hands of patients.
Adding to the excitement, on June 13, 2024, Celltrion announced encouraging Phase III results for CT-P47, a biosimilar candidate referencing RoActemra® for patients with moderate-to-severe rheumatoid arthritis, during the Annual European Congress of Rheumatology (EULAR) 2024. In a Phase III comparative clinical trial involving 471 patients, CT-P47 demonstrated equivalent efficacy and a comparable safety and immunogenicity profile to the reference product, tocilizumab. Participants received either CT-P47 or the reference tocilizumab at a dose of 8 mg/kg every four weeks for the initial 20 weeks. At week 24, those on tocilizumab were re-randomized to either continue with the reference drug or transition to CT-P47 for the remainder of the 48-week trial. This development signifies a vital step forward in providing alternatives to existing treatments and reflects the dynamic progress being made in the RA therapeutic landscape.
Another contender, Imvotamab from IGM Biosciences, adds another layer of excitement to the rheumatoid arthritis pipeline. This bispecific T cell engaging IgM antibody has a unique design, featuring ten binding units for CD20, allowing it to engage B cells with unprecedented avidity. Currently undergoing evaluation in a Phase-Ib trial for severe rheumatoid arthritis, Imvotamab has demonstrated in vitro superiority over rituximab, particularly in its ability to deplete low CD20-expressing B cells. This could herald a new era in the treatment of autoimmune diseases mediated by B cells, showcasing the innovative spirit driving research in this field.
The list of emerging therapies doesn’t stop there. SM03, a promising candidate from SinoMab Bioscience, and Telitacicept (RC18) from RemeGen Co. Ltd., are also vying for attention in the RA landscape. IRL201805, developed by Immune Regulation, and ORYN 1001 from Oryn Therapeutics are further testament to the rich tapestry of research and development in this arena. The excitement surrounding these candidates underscores a collective effort to tackle rheumatoid arthritis from multiple angles, offering a glimpse into a future where patients might have access to a variety of treatment options tailored to their unique needs.
The sheer volume of activity in the rheumatoid arthritis pipeline is staggering, with over 75 companies working on more than 80 drugs. Key players include OncoOne, Sonoma Biotherapeutics, Teijin Pharma, Eli Lilly and Company, SynAct Pharma, Jiangsu Hengrui Medicine Co., I-Mab Biopharma US Limited, Celon Pharma SA, Lipum, Incannex Healthcare Ltd, AbbVie, Lynk Pharmaceuticals Co., Ltd, Sanofi, and Chia Tai Tianqing Pharmaceutical Group Co., Ltd. Each company is a crucial part of the tapestry, contributing to a hopeful narrative that promises to change the face of RA treatment.
As we look toward the future, the combination of established rheumatoid arthritis treatments and innovative new therapies for rheumatoid arthritis paints an optimistic picture for those living with the condition. With each trial, each discovery, and each step forward, the potential for improved patient with rheumatoid arthritis outcomes grows. The evolution of rheumatoid arthritis treatment is not just a story of medications for rheumatoid arthritis and therapies; it’s a narrative of hope, resilience, and the relentless pursuit of a life free from pain and limitation.
Whether through novel treatment for rheumatoid arthritis, such as biologics or the next generation of JAK inhibitors, or through lifestyle changes that complement traditional rheumatoid arthritis medical procedures, there is a clear path toward better management and an improved quality of life. Though we may not yet have a definitive answer to the question of how to cure rheumatoid arthritis permanently, advancements in research continue to give us reason to hope that one day this disease will no longer limit the lives of those affected by it.

Downloads
Article in PDF
Recent Articles
- Duchenne Muscular Dystrophy: Advances, Challenges, and the Future of Treatment
- Global atrial fibrillation market is expected to grow to $12.5 billion by 2020
- Hereditary Angioedema (HAE) – Active drug pipeline
- Novo petitions FDA;J&J invests; Xarelto’s trial; Roche’s Tecentriq
- Thrombolytic Sciences’ trial; NurOwn for ALS; Biosimilar receives approval; Trelegy Ellipta for t...