Ono’s ROMVIMZA Triumph Sparks Battle with Daiichi Sankyo’s TURALIO in TGCT Space
Mar 03, 2025
Ono Pharmaceuticals received FDA approval on 14 February 2025 for vimseltinib, a treatment for tenosynovial giant cell tumor (TGCT), which will be sold under the brand name ROMVIMZA. This approval positions Ono in direct competition with fellow Japanese pharmaceutical company Daiichi Sankyo in the TGCT market.
Daiichi’s drug, TURALIO, is approved for adult TGCT patients experiencing severe morbidity or functional limitations for whom surgery is not a viable option. In its latest quarterly report for Q3 of the 2024 fiscal year, Daiichi reported a nearly 25% increase in TURALIO sales, reaching ¥5.1 billion (approximately $33.6 million).
Similar to TURALIO, which became the first FDA-approved treatment for TGCT in 2019, ROMVIMZA is also a colony-stimulating factor 1 receptor (CSF1R) inhibitor. ROMVIMZA is a kinase inhibitor that functions by inhibiting the CSF1 receptor, thereby hindering the growth of cells expressing this protein. In TGCT, this mode of action may help restrict the spread of the tumor mass.
Downloads
Article in PDF
Recent Articles
- FDA Grants Priority Review to Merck’s Application for KEYTRUDA Plus Padcev; Roche and Carmot Ther...
- EU Approves Galderma’s NEMLUVIO for Atopic Dermatitis and Prurigo Nodularis; GSK’s Penmenvy Wins ...
- Ipsen and Skyhawk Therapeutics Partnership; SynOx Therapeutics’ Phase III Trial; Roche’s Alecensa...
Ono Pharmaceuticals recently acquired ROMVIMZA after purchasing Deciphera Pharmaceuticals for $2.4 billion last year, making the U.S.-based company its subsidiary. At the time, Ono’s CEO, Gyo Sagara, highlighted the acquisition as a strategic move to expand the company’s targeted oncology portfolio, accelerate its business growth in the U.S. and Europe, and enhance its kinase drug discovery capabilities.
To compete with TURALIO’s first-mover advantage, Ono is positioning ROMVIMZA as a more convenient TGCT treatment option. Unlike Daiichi’s drug, which requires twice-daily dosing, ROMVIMZA is administered just twice a week, with a minimum of 72 hours between doses. Additionally, TURALIO comes with a boxed warning for hepatotoxicity due to its potential to cause serious or fatal liver damage, whereas ROMVIMZA does not carry such a warning.
The approval of ROMVIMZA marks a significant advancement, offering a well-tolerated and effective treatment option for individuals suffering from TGCT. According to Dr. Hans Gelderblom, Chair of the Department of Medical Oncology at Leiden University Medical Center, TGCT significantly impacts patients’ lives by causing pain, restricted mobility, and stiffness. The Phase III MOTION study demonstrated ROMVIMZA’s ability to shrink tumors while also being the first well-tolerated treatment to show substantial improvements in key quality-of-life measures—without the liver toxicity observed with other approved TGCT therapies. This makes ROMVIMZA a unique treatment with the potential to address critical unmet needs in the TGCT community.
The FDA’s approval of ROMVIMZA represents a major breakthrough for TGCT patients and has the potential to establish a new standard of care for those in whom surgery could lead to further functional impairment or severe complications. Ryota Udagawa, President and CEO of Deciphera Pharmaceuticals, emphasized that this milestone is not only crucial for patients but also for the company, as ROMVIMZA is the second approved therapy developed through Deciphera’s proprietary switch-control kinase inhibitor platform. He expressed gratitude to the patients, caregivers, healthcare providers, and the teams at Deciphera and Ono for their dedication in advancing this transformative treatment, which they are eager to make available to those in need.
TGCT comprises a group of rare tumors that, while generally not life-threatening, can significantly impact the joints and surrounding tissues, potentially causing severe disability. If untreated, TGCT may lead to the need for amputation. Among the 7MM, the United States had the highest prevalence of tenosynovial giant cell tumor, accounting for approximately 45% of total cases.
In 2023, localized TGCT in the U.S. was most commonly found in the digits, representing around 75% of cases. Depending on whether the tumor is localized or diffuse, giant cell tumors can also occur in the knee, ankle, hip, and other areas. According to DelveInsight analysts, diffuse TGCT was most frequently observed in the knee, with an estimated 30,000 cases in the U.S. in 2023.
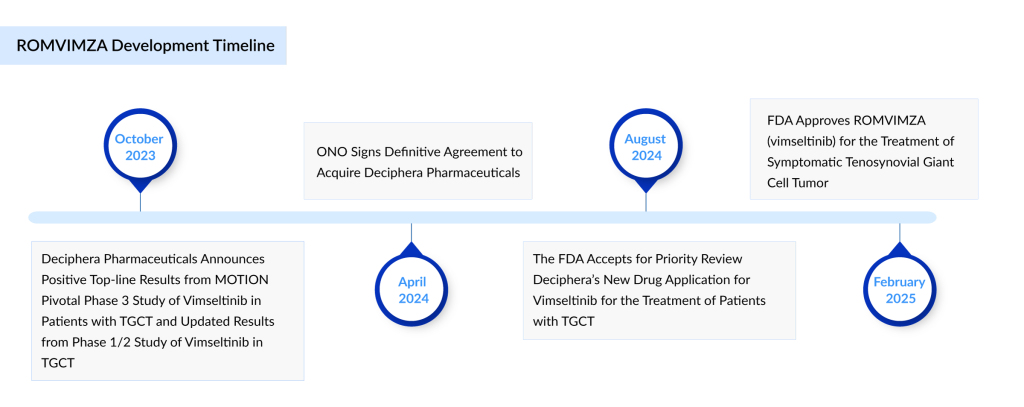
The FDA approved ROMVIMZA based on the efficacy and safety findings from the pivotal Phase III MOTION study in patients with TGCT who were not eligible for surgery and had not received prior anti-CSF1/CSF1R therapy (though previous treatment with imatinib or nilotinib was permitted), as well as data from the Phase I/II TGCT clinical trial. In the MOTION study, ROMVIMZA achieved a statistically significant and clinically meaningful overall response rate (ORR) at Week 25 in the intent-to-treat (ITT) population, as determined by blinded independent radiologic review (BIRR) using RECIST v1.1 criteria, compared to placebo (40% vs. 0%, p <0.0001).
The primary endpoint was further supported by significant improvements in active range of motion, patient-reported physical function, and pain reduction in the vimseltinib-treated group versus placebo at Week 25. The safety profile of ROMVIMZA remains manageable and aligns with previously reported Phase I/II trial results. In July 2024, Ono announced that the European Medicines Agency (EMA) had accepted and is reviewing the marketing authorization application (MAA) for ROMVIMZA as a treatment for patients with TGCT.
In 2019, Daiichi’s TURALIO received FDA approval based on a 39% overall response rate at Week 25, compared to 0% for the placebo, in TGCT patients for whom surgery was not advised. Among those monitored for at least six months, 96% maintained their responses for a minimum of six months. ROMVIMZA offers a key advantage in terms of convenience. It is administered at 30 mg twice weekly, whereas TURALIO requires a dosage of 250 mg twice daily. Both treatments are taken orally.
Additionally, due to concerns over “serious and potentially fatal liver injury,” which is flagged with a black box warning, TURALIO is only accessible through a restricted Risk Evaluation and Mitigation Strategy (REMS) program. During its phase III TGCT clinical trial, enrollment was halted early by the data monitoring committee after observing cases of cholestatic liver toxicity.
In contrast, the MOTION trial found no signs of cholestatic hepatotoxicity or drug-induced liver injury with ROMVIMZA. However, 10% of patients on ROMVIMZA experienced treatment-emergent grade 3 or 4 elevated blood creatine phosphokinase levels, an indicator of muscle injury or stress. Unlike TURALIO, ROMVIMZA’s label does not carry a boxed warning or require a REMS program.
ROMVIMZA and TURALIO may soon encounter a new competitor, Merck KGaA’s pimicotinib. Pimicotinib is an experimental, orally administered small-molecule inhibitor that selectively and effectively targets the colony-stimulating factor-1 receptor. Developed by Abbisko, it is currently under investigation. In December 2023, Abbisko Therapeutics agreed with Merck, granting Merck an exclusive license to commercialize pimicotinib-based products for all indications in mainland China, Hong Kong, Macau, and Taiwan, with an option to expand to global markets, including the US.
Pimicotinib has received Breakthrough Therapy Designation (BTD) from both the China National Medical Products Administration (NMPA) and the FDA, as well as Priority Medicine (PRIME) designation from the European Medicines Agency (EMA) for treating patients with TGCT who are not eligible for surgery.
In November 2024, Merck announced that the Phase III MANEUVER trial for pimicotinib, conducted by Abbisko Therapeutics, achieved its primary objective. The trial demonstrated a significant improvement in the objective response rate (ORR) for patients with tenosynovial giant cell tumor. At week 25, the ORR for pimicotinib was 54.0%, compared to 3.2% for the placebo (p<0.0001).
The anticipated launch of Merck KGaA’s pimicotinib will not only boost the TGCT therapeutic segment but also give fierce competition to both Ono’s ROMVIMZA and Daiichi Sankyo’s TURALIO. It will be interesting to see how both companies will retain their position in the competitive TGCT treatment landscape.

Downloads
Article in PDF
Recent Articles
- Ipsen and Skyhawk Therapeutics Partnership; SynOx Therapeutics’ Phase III Trial; Roche’s Alecensa...
- FDA Grants Priority Review to Merck’s Application for KEYTRUDA Plus Padcev; Roche and Carmot Ther...
- EU Approves Galderma’s NEMLUVIO for Atopic Dermatitis and Prurigo Nodularis; GSK’s Penmenvy Wins ...



