J&J’s SPRAVATO Achieves Milestone with Monotherapy Approval in Depression Treatment
Feb 21, 2025
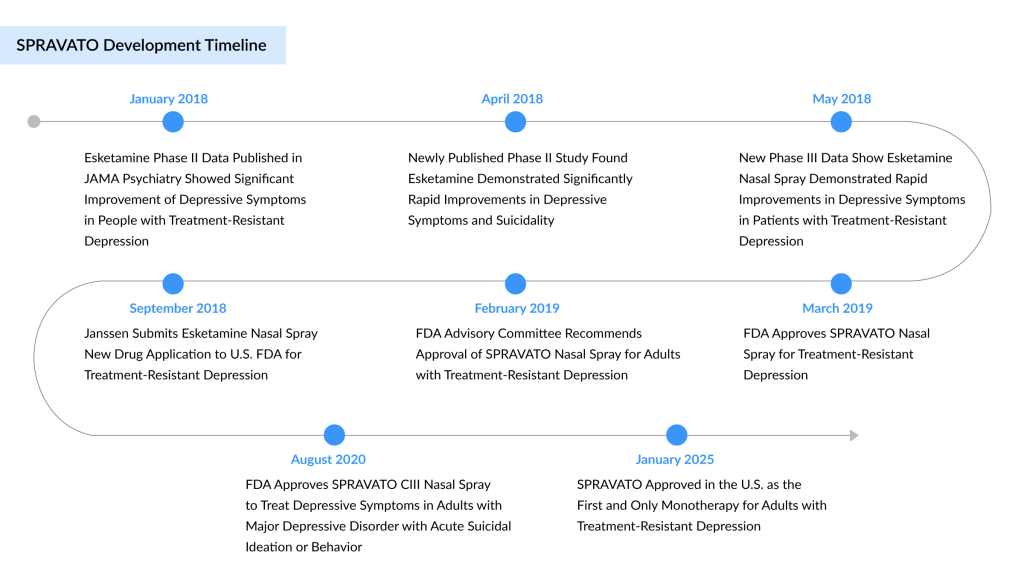
Johnson & Johnson’s SPRAVATO, which is already on track to become a blockbuster drug, has received a significant boost with the FDA’s approval for its use as a standalone treatment for major depressive disorder (MDD). Initially approved in 2019, SPRAVATO was authorized for use alongside an oral antidepressant in patients who did not respond to other antidepressant treatments.
SPRAVATO functions as a non-selective, non-competitive antagonist of the NMDA receptor, impacting glutamate pathways in the brain. While its precise mechanism of antidepressant action is not fully understood, it has been shown to have an effect. In July 2024, Johnson & Johnson filed a supplemental New Drug Application (sNDA) with the US FDA, seeking approval for esketamine (SPRAVATO) CIII nasal spray as a standalone treatment for adults with treatment-resistant depression, aiming to expand its approved uses.
In August 2016, the US FDA awarded a second Breakthrough Therapy Designation (BTD) to intranasal esketamine, recognizing its potential to treat patients with major depressive disorder who are at imminent risk of suicide, as well as those with treatment-resistant depression. In 2020, the U.S. regulatory authority granted additional approval for SPRAVATO to be used in patients with MDD who have acute suicidal thoughts or behaviors.
Downloads
Article in PDF
Recent Articles
- Major Depressive Disorder: Unveiling Market Moves and Commercial Breakthroughs
- Caristo Wins FDA Clearance for AI Solution to Prevent Heart Attacks; Lindus Health and Sooma Medi...
- Evaluating the Role of Digital Therapeutics as an Alternative to Conventional Therapies for Depre...
- Sleep Aids Market: Examining Cutting-Edge Technologies and Major Breakthroughs
- Relievant Medsystems Launched Ablation System; Thermo Fisher’s Reproductive Health Assays; EarliT...
Supported by over a decade of research and nearly six years of real-world data, SPRAVATO has emerged as a groundbreaking treatment for many individuals with TRD. It has been shown to alleviate depression symptoms within just 24 hours and significantly delay relapse for those who continue therapy. As of now, SPRAVATO has received approval in 77 countries and has been administered to over 140,000 patients globally.
Major depressive disorder is a prevalent psychiatric condition, affecting an estimated 21 million adults in the U.S. Approximately one-third of adults with MDD do not respond to oral antidepressants alone, severely impacting their quality of life. The economic burden of MDD is substantial, with nearly half of it stemming from treatment-resistant depression.
“Managing treatment-resistant depression can be highly complex, particularly for patients who either do not respond to oral antidepressants or struggle with their side effects. For years, healthcare providers have had limited options to help these patients achieve meaningful symptom relief,” said Bill Martin, Ph.D., Global Therapeutic Area Head, Neuroscience, Johnson & Johnson Innovative Medicine. “With SPRAVATO now available as a standalone treatment, patients may see improvements in depressive symptoms as soon as 24 hours and at 28 days—without relying on daily oral antidepressants.”
Depression is a prevalent mental health condition affecting around 280 million people globally. In the United States, about 21 million adults have experienced at least one major depressive episode. Nearly one-third of individuals with major depressive disorder do not respond adequately to oral antidepressants alone, classifying them as having treatment-resistant depression, which is characterized by an insufficient response to two different oral medications.
As per DelveInsight analysis, the total diagnosed prevalent cases of MDD in the 7MM were found to be approximately 44 million cases in 2023, which is expected to increase during the forecast period (2025-2034). In 2023, the US accounted for the highest diagnosed prevalent cases of MDD in the 7MM with approximately 22 million cases.
As per DelveInsight analysis, there were 6.4 million prevalent cases of TRD across the 7MM in 2023, and this figure is projected to rise by 2034. TRD severely affects the lives of those who suffer from it and carries one of the highest economic burdens among psychiatric disorders. Patients typically go through multiple oral medications, enduring a 4-6 week wait for possible relief. Even after trying a third oral antidepressant, around 86% of patients still do not reach remission.
Earlier in January 2025, Neumora Therapeutics’ highly anticipated drug, navacaprant, failed to demonstrate a significant reduction in the severity of depressive episodes in a late-stage trial for major depressive disorder. Similarly, in December 2024, Relmada had to halt two Phase III trials of its MDD candidate, REL-1017, following an unfavorable futility review.
Perhaps the most significant setback was Biogen and Sage’s inability to secure FDA approval for their oral treatment, ZURZUVAE, in MDD. While the drug was approved for postpartum depression treatment in August 2023, regulators determined that the companies had not provided “substantial evidence of effectiveness” for its use in the broader MDD market. With this regulatory approval, SPRAVATO is now authorized for use on its own in adult patients who have not responded adequately to at least two oral antidepressant treatments.
This approval, granted after an FDA Priority Review, is based on positive findings from a randomized, double-blind, multicenter, placebo-controlled study. The study demonstrated that SPRAVATO alone led to a rapid and significantly greater improvement in the Montgomery-Åsberg Depression Rating Scale (MADRS) total score compared to placebo. A post-hoc analysis further showed numerical improvements across all 10 MADRS items by day 28. By week 4, remission (MADRS total score ≤ 12) was achieved by 22.5% of patients receiving SPRAVATO, compared to 7.6% in the placebo group. The safety profile of SPRAVATO as a standalone treatment aligned with existing clinical and real-world data when used alongside an oral antidepressant, with no new safety concerns identified.
Due to the potential for serious adverse effects such as sedation, dissociation, respiratory depression, abuse, and misuse, SPRAVATO is available only through a restricted program—the SPRAVATO Risk Evaluation and Mitigation Strategy (REMS) Program—to ensure its safe and appropriate use.
“For over six years, I have personally witnessed the meaningful impact SPRAVATO can have on patients’ lives,” said Gregory Mattingly, M.D., President of Midwest Research Group and Founding Partner of St. Charles Psychiatric Associates. “With its availability as a monotherapy, healthcare providers now have greater flexibility to tailor treatment plans to individual needs, allowing patients to experience the benefits of SPRAVATO as early as 24 hours and up to day 28, without requiring a daily oral antidepressant.”
This approval has also spurred competition in the TRD therapeutic segment. Several companies such as COMPASS Pathways, SAGE Therapeutics, Biogen, Luye Pharma, Relmada Therapeutics, Minerva Neurosciences, BioLite, ABVC BioPharma, Denovo Biopharma, Atai Life Sciences, and others are actively working with their lead assets to grab a significant market share in the TRD and MDD market space.

Downloads
Article in PDF
Recent Articles
- Caristo Wins FDA Clearance for AI Solution to Prevent Heart Attacks; Lindus Health and Sooma Medi...
- Role of Digital Therapeutics (DTx) in Mental Health Management
- Sage seeks to save depression drug; Regeneron aims clinical trial; J&J sets sights for COVID...
- How to Cure Treatment-resistant Depression?
- Watch Out For Top Pipeline Therapies Making An Impact In The Bipolar Depression Market