Acute Ischemic Stroke
Mar 10, 2025
Roche’s TNKase — First New Acute Ischemic Stroke Drug in Nearly 30 Years
For nearly three decades, Genentech has been the sole provider of an approved medication for acute stroke treatment. On 03 March 2025, the Roche subsidiary expanded its stroke treatment portfolio with FDA approval for TNKase (tenecteplase). TNKase (tenecteplase) is a thrombolytic, clot-dissolving tis...
Read More...
Nov 05, 2024
Prolong’s PP-007 Fast-Tracked for Stroke; FDA Expands JYLAMVO Pediatric Approval; Corcept’s Cushing’s Drug Shows Positive Phase III Results; ESSA Halts Phase II Study of Masofaniten for Prostate Cancer; SCEMBLIX Approved for Leukemia
Prolong Pharmaceuticals Secures FDA Fast Track for PP-007 in Stroke Therapy Prolong Pharmaceuticals, LLC, a clinical-stage biopharmaceutical company, announced that its investigational therapy, PP-007 (PEGylated carboxyhemoglobin, bovine), has been granted Fast Track designation by the FDA for the treatment of a...
Read More...
Jun 20, 2024
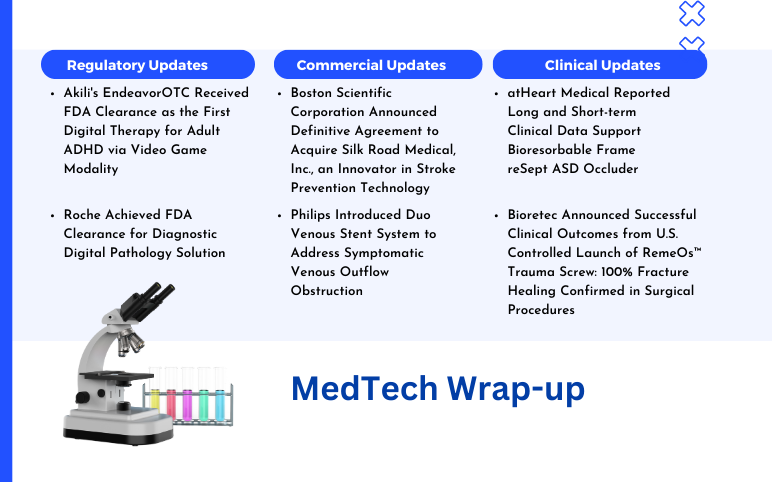
Boston Scientific Corporation Agreement to Acquire Silk Road Medical; Philips Introduced Duo Venous Stent System; Akili’s EndeavorOTC Received FDA Clearance; Roche Achieved FDA Clearance; atHeart Medical Reported Long and Short-term Clinical Data; Bioretec Announced Successful Clinical Outcomes
Boston Scientific Corporation Announced Definitive Agreement to Acquire Silk Road Medical, Inc., an Innovator in Stroke Prevention Technology On June 18, 2024, Boston Scientific Corporation, a global leader in medical technologies, announced the formal agreement to acquire Silk Medical, Inc., a medical device co...
Read More...
Feb 08, 2024
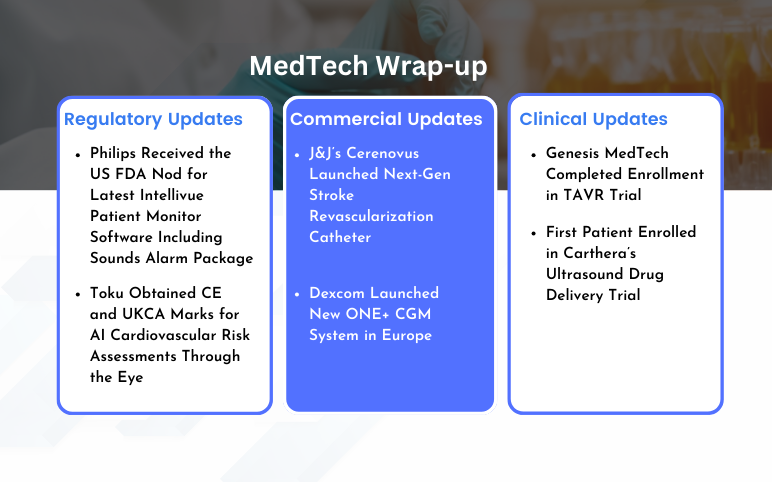
Cerenovus Launched Next-Gen Stroke Revascularization Catheter; Dexcom Launched New ONE+ CGM System; Philips Received the US FDA Nod for Latest Intellivue Patient Monitor Software; Toku Obtained CE and UKCA Marks for AI Cardiovascular Risk Assessments; Genesis MedTech Completed Enrollment in TAVR Trial; Carthera’s Ultrasound Drug Delivery Trial
J&J’s Cerenovus Launched Next-Gen Stroke Revascularization Catheter On February 7, 2024, Johnson & Johnson MedTech’s Cerenovus announced the launch of its next-generation CereGlide 71 intermediate catheter with TruCourse indicated for the revascularization of patients suffering from acute ischemic stroke...
Read More...
Mar 06, 2023
Wider Administration Window – Need of the Moment for Acute Ischemic Stroke Patients
Acute ischemic stroke, contributing up to 80% of all stroke cases, is among the leading causes of death and morbidity, leaving around 50% of stroke survivors disabled worldwide. It is caused due to the blockage of an artery resulting in reduced blood flow to the brain that damages and interrupts brain functions. Hi...
Read More...


-Agonist.png)

