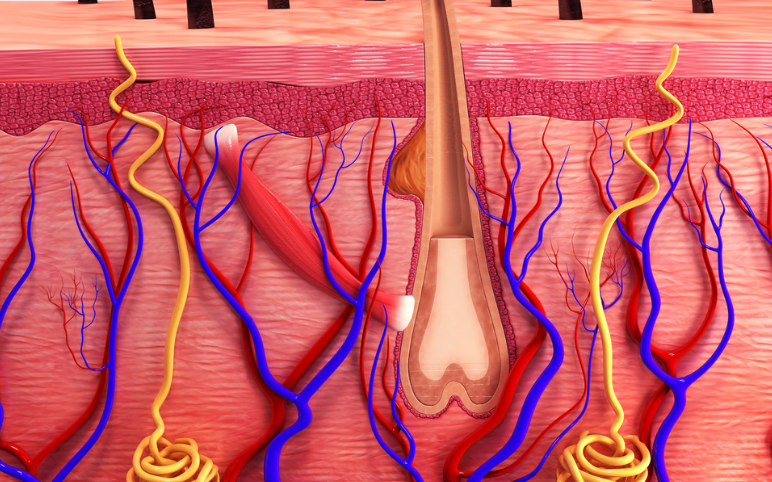
alopecia areata
Dec 30, 2024
6 Emerging Treg Cell-based Therapies Shaping the Future of Immunotherapy
Treg (regulatory T-cell) therapies utilize the immunosuppressive abilities of regulatory T-cells to address various conditions, such as autoimmune diseases, inflammatory disorders, and transplant rejection. These treatments take advantage of Tregs’ natural role in maintaining immune balance by suppressing overactiv...
Read More...
Jun 27, 2023
FDA Approves Jardiance for Type 2 Diabetes; FDA Approves Pfizer’s LITFULO for Alopecia Areata; Sarepta Therapeutics’s ELEVIDYS Approval; Tonix Pharmaceuticals to Acquire Two Migraine Products from Upsher-Smith; FibroGen’s Phase 3 ZEPHYRUS-1 Study of Pamrevlumab; FDA Orphan Drug Designation to ERAS-801 for Malignant Glioma
FDA Approves Jardiance for the Treatment of Type 2 Diabetes in Children 10 Years and Older Boehringer Ingelheim and Eli Lilly and Company announced that the FDA has approved Jardiance® (empagliflozin) 10 mg and 25 mg tablets to decrease blood sugar together with diet and exercise in children 10 years and older w...
Read More...
Sep 09, 2022
Alopecia Areata Treatment: Which Pipeline Therapy Will Steal the Spotlight?
Currently, there is no cure and no FDA-approved treatments for alopecia. Thus, the alopecia areata treatment market is completely dominated by off-label drug therapies. Depending on the extent of hair loss and age, there are a variety of alopecia areata treatment options are available. The main goal of treatment is...
Read More...
Feb 02, 2021
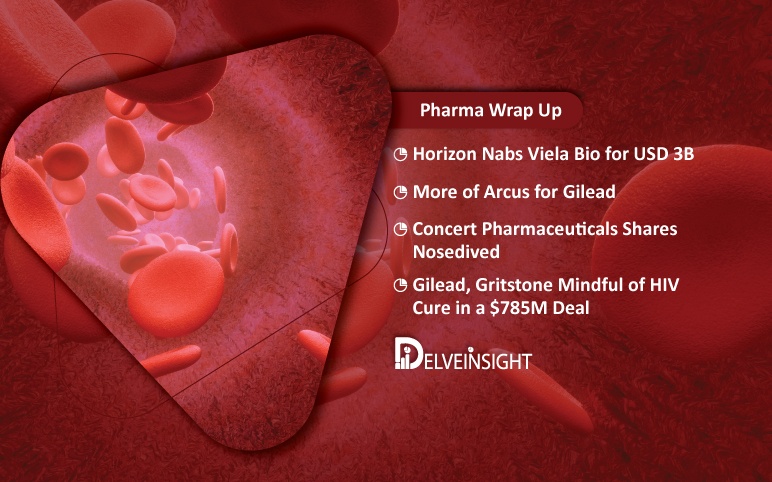
Horizon, Viela Deal; Concert Schizo Drug Flop; Gilead Deals with Arcus and Gritstone
Horizon Therapeutics Bolsters its Rare Disease Portfolio in a $3B Viela Bio Buyout Horizon Therapeutics has announced to acquire Viela Bio for about $3.1 billion to augment its portfolio and pipeline of treatments for rare diseases. The shares of Viela Bio skyrocketed more than 52% in premarket trading after the...
Read More...
Mar 17, 2020
Moderna’s COVID-19 vaccine; AcelRx buys TetraPhase; Breakthrough designation to Baricitinib
Moderna announces dozing of first patient with its mRNA1273 vaccine The pharmaceutical company, Moderna, has been developing a vaccine for the treatment of COVID-19. The vaccine is an mRNA-1273 is developed by scientist at NAID, in collaboration with Moderna, and the manufacturing of the vaccine for Phase I clin...
Read More...
Oct 17, 2016
JAK Inhibitors: New Lifeline for Hair Loss Treatment
Janus Kinase (JAK) inhibitors are the ones that inhibit the functional activity of enzymes that belong to Janus Kinase family such as JAK1, JAK2, JAK3 and TYK2 by interfering in the JAK-STAT signaling pathway. JAK Inhibitors are used for the treatment purpose in cancer and inflammatory diseases like rheuma...
Read More...



-Agonist.png)

