Alzheimer’s disease
Oct 04, 2022
Zealand Pharma’s Phase III Results of Glepaglutide; FDA Approves Amylyx’s ALS Drug Relyvrio; Novo Nordisk and Ventus Therapeutics Signs Licencing Deal; FDA Approves Futibatinib; Sarepta Files Duchenne Muscular Dystrophy for FDA Approval; Biogen and Eisai’s Lcanemab Phase III Study
Zealand Pharma Announces the Positive Topline Results from its Phase III trial of Glepaglutide Zealand Pharma A/S, a biotech company specializing in peptide-based medicines, announced positive topline results from its phase III trial of glepaglutide. In the evenly randomized double-blind trial, 106 patients with...
Read More...
Jul 12, 2022
Novo Nordisk’s Concizumab for Hemophilia; AbbVie Ends its Alliance with Alector; ADC Therapeutics and Sobi Enters in Exclusive Licensing Deal; BMS’ Opdivo Gets NHS Use; Merck to Acquire Seagen; FDA Priority Review to Roche’s Lunsumio; AstraZeneca to Acquire TeneoTwo; FDA Orphan Drug Designation to PBI-200
Novo Nordisk Reports Phase III Results of Concizumab Drug for Hemophilia A or B Novo Nordisk has announced Phase III results for their concizumab drug for hemophilia A or B, demonstrating efficacy in preventing bleeding events and paving the way for regulatory filings later this year. Concizumab, an anti-tissue ...
Read More...
Jul 07, 2022
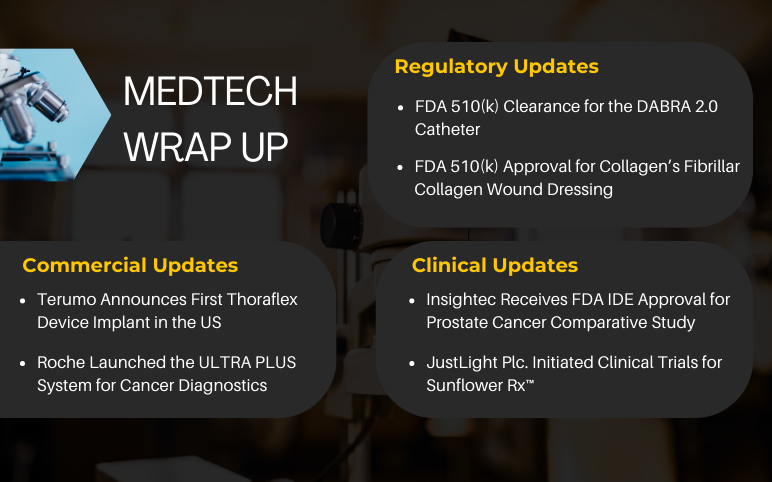
Collagen Matrix FDA 510(k) approval for Fibrillar Collagen Wound Dressing; Roche’s cancer diagnostics ULTRA PLUS system launch; Insightec IDE Approval for Prostate Cancer; JustLight plc. trial of Sunflower Rx for Alzheimer’s disease; Thoraflex Hybrid Device Implantation in the United States; FDA 510(k) Clearance for the DABRA 2.0 Catheter
Collagen Matrix received FDA 510(k) approval for Fibrillar Collagen Wound Dressing On June 29, 2022, Collagen Matrix, Inc., a leader in regenerative medicine, a global manufacturer of collagen and other biomaterial-based medical devices, and Linden Capital Partners portfolio company announced FDA 510(k) Clearan...
Read More...
Jun 21, 2022
Biogen terminates ALS Pact with Karyopharm; AbbVie’s Immunological Drug Skyrizi; NICE Backs Astellas’ Oral Therapy Evrenzo; Roche’s Crenezumab Fails in Clinical Trial; FDA Grants Fast Track Designation to Dianhydrogalactitol; Sierra Oncology Submits NDA for Momelotinib
Biogen terminates USD 217 Million ALS Pact with Karyopharm Biogen has backed out of the four-year-old partnership with Karyopharm on a drug candidate for the neurological disease amyotrophic lateral sclerosis, which could have cost the US biotech up to USD 217 million. The 2018 agreement that granted Biogen righ...
Read More...
Apr 26, 2022
Coherus Biosciences’ Toripalimab; Keymed’s CMG901; Biogen Alzheimer’s Drug Aduhelm; Vicore’s Digital Therapeutics Study; Cassiopea’s Acne Treatment Winlevi; Samsung Biologics Acquires Samsung Bioepis; Roche’s Giredestrant; Ashai Kasei Acquires Bionova
FDA Grants Orphan Drug Designation to Coherus Biosciences’ Toripalimab for Small Cell Lung Cancer The FDA granted orphan drug status to Coherus Biosciences’ Toripalimab (TuoYi) based on data from the phase 3 JUPITER-08 study in patients with extensive-stage Small-cell Lung Cancer (ES-SCLC). Patients in the curre...
Read More...
Apr 25, 2022
Rise In Bispecific Antibodies Utilization As Antibody Therapeutics
Bispecific Antibodies (BsAbs) are antibodies that contain two different antigens or have two different epitopes on the same antigen. It is observed for Bispecific Antibodies in clinical trials that the therapeutic effects of these are considerably higher than those of monoclonal antibodies (MoAbs). There are a vast...
Read More...
Mar 22, 2022
Bristol Myers’ Opdivo combo Opdualag for Melanoma; Biogen’s Aduhelm; Marinus’ Ztalmy for CDKL-5 Deficiency Disorder; Merck’s Keytruda + Lynparza; Vitaris’s Breyna; Moderna’s Second COVID-19 Booster Shot; Takeda’s Exkivity; BMS’s First LAG-3 Checkpoint Inhibitor
NHS Grants Fast Track Access to Takeda’s Exkivity Takeda has secured UK approval for its lung cancer therapy Exkivity, with an NHS access deal that could see it prescribed to patients within the next few weeks. The Medicines and Healthcare products Regulatory Agency has granted conditional marketing authorisa...
Read More...
Mar 01, 2022
GSK’s Covifenz; Idorsia’s Quviviq; GSK’s ZEJULA; EMA Expands its Nod for BMS, Eli Lilly, and Novartis Drugs; Cantex Secures Global Licence to Develop Azeliragon; Biocon Acquires Viatris’ Biosimilar Business
GSK’s Covifenz, A Plant-Based COVID Vaccine, Receives First Approval The recombinant COVID-19 vaccine developed by Medicago, now known as Covifenz, has received approval in Canada, the company's home country. Covifenz employs Coronavirus-Like Particle (CoVLP) technology, with the vaccine consisting of recombinan...
Read More...
Feb 08, 2022
Biogen’s Aduhelm; FDA Approves Sanofi’s Enjaymo; NHS & Orchard Signs a Deal; Bristol’s Breyanzi & Abecma; Pharming’s Leniolisib; Eli Lilly’s Verzenio Trial; UCB’ Zilucoplan Trials Result; Eli Lilly’s Alzheimer’s Drug Donanemab
Biogen's Aduhelm Marketing and Approval are Under Scrutiny in New FTC and SEC Investigations Biogen can't seem to get a break when it comes to the Alzheimer's disease drug Aduhelm. Along with limited sales and a constraining Medicare coverage plan, the medicine is now facing additional scrutiny as part of a pair...
Read More...
Jan 20, 2022
Suneva Medical-Viveon Health merger update; Smith+Nephew acquires Engage Surgical; Abiomed’s preCARDIA technology; Medtronic’s DTM Spinal Cord Stimulation; Endo Tools Therapeutics’s Endomina System; Diadem’s AlzoSure predict prognostic blood test
Suneva Medical Inc. inked a merger agreement with Viveon Health On January 12, 2022, Suneva Medical Inc., a leading regenerative aesthetics company, and Viveon Health Acquisition Corp., a particular purpose acquisition company, entered into a definitive merger agreement to establish a new regenerative aesthetics...
Read More...









-Agonist.png)

