Atrial Fibrillation
Dec 03, 2024
FDA Grants Fast Track for Lin BioScience’s LBS-007; Alnylam’s AMVUTTRA sNDA Under Review; FDA Approves RAPIBLYK for Atrial Fibrillation; Applied Therapeutics Receives CRL for Govorestat in Galactosemia; R289 Gets Fast Track for Lower-Risk MDS.
Lin BioScience Receives FDA Fast Track Designation for LBS-007 Lin BioScience, a clinical-stage biopharmaceutical company focused on developing innovative therapies for acute leukemia, announced that its lead pipeline candidate, LBS-007, has been granted Fast Track Designation by the FDA for the treatment of acu...
Read More...
Jan 04, 2024
HR Pharmaceuticals Announced Collaboration with Poiesis Medical; Enovis Acquired Lima Corporate; AnX Robotica Announced FDA Clearance for ProScan; FDA Breakthrough Device Designation for CGBio’s ‘NOVOSIS PUTTY’; EndoSound Received 510(k) Clearance for Breakthrough EVS Innovation; Boston Scientific Initiated AVANT GUARD Clinical Trial
HR Pharmaceuticals, Inc. Received Exclusive Commercialization Rights to Poiesis Medical’s Dual Balloon Catheter Technology in North America On December 28, 2023, HR Pharmaceuticals entered into a collaboration with Poiesis Medical LLC, to license Poiesis's Dual Balloon Catheter (Duette™). According to the terms ...
Read More...
Dec 21, 2023
Integra to Buy J&J’s Acclarent; B. Braun Launches the CARESITE Micro Luer Access Devic; FDA Approves Medtronic’s Novel PulseSelect Pulsed Field Ablation System; FDA Clearances to Perfuze’s Novel Neurovascular Aspiration and Access Catheters for Stroke Treatment; ShiraTronics Successfully Implants First Migraine System; Sight Sciences Announced the Six-Month Results For Heat Therapy Device
Integra LifeSciences to Buy Johnson & Johnson’s Acclarent and its ENT Tech On December 13, 2023, Integra LifeSciences entered into a definitive agreement to acquire Acclarent from Johnson & Johnson MedTech. Acclarent strengthens Integra's position in the ENT treatment market. Acclarent is a component of ...
Read More...
Nov 21, 2023
Key Updates on Phase 1 Trial of AB-1005 Gene Therapy for Multiple System Atrophy-Parkinsonian Type; European Commission Approves EBGLYSS; Bayer Stopped OCEANIC-AF Study; Pfizer and Astellas’ XTANDI Approved by FDA; FDA Orphan Drug Designation to Epic Bio’s EPI-321; FDA Fast Track Designation to Chemomab’s CM-101 for PSC
AskBio Announces First Patient Randomized in Phase 1 Trial of AB-1005 Gene Therapy for Multiple System Atrophy-Parkinsonian Type Asklepios BioPharmaceutical, Inc., a gene therapy firm fully owned and independently operated under Bayer AG, announced the initiation of the Phase I REGENERATE MSA-101 clinical trial ...
Read More...
Nov 07, 2023
New Asundexian Phase III Study Result; Zibotentan/Dapagliflozin Combination Demonstrated Significant Albuminuria Reduction Chronic Kidney Disease; Orphan Drug Designation to Rhenium Obisbemeda; FDA Approves Merck’s KEYTRUDA Plus Gemcitabine and Cisplatin for Biliary Tract Cancer; Orphan Drug Designation to Ayala’s AL102; GARDP Announces Successful Phase 3 Trial of of Uncomplicated Gonorrhea
Positive Results Announced in Largest Pivotal Phase 3 Trial of a First-in-Class Oral Antibiotic to Treat Uncomplicated Gonorrhea The Global Antibiotic Research & Development Partnership (GARDP), in partnership with Innoviva, Inc. (Nasdaq: INVA), had announced a significant milestone. They revealed that zolif...
Read More...
Feb 02, 2023
Alma Announced the Launch of Alma Duo; Phillips-Medisize Launched a Pen Injector Platform; FDA Approved Abbott’s Spinal Cord Stimulation; Axonics Received FDA Approval for Rechargeable Sacral Neuromodulation; SELUTION SLR Coronary Sirolimus DEB Study Enrolls First Patient; AtriCure LeAAPS Clinical Trial
Alma announced the Launch of Alma Duo- an Advanced Solution for Sexual Wellness On January 26, 2023, Alma, the core subsidiary of Sisram Medical and one of the leaders of energy-based medical and aesthetics solutions, launched its new sexual wellness product, Alma Duo. Alma Duo is a cutting-edge, safe, non-in...
Read More...
Aug 04, 2022
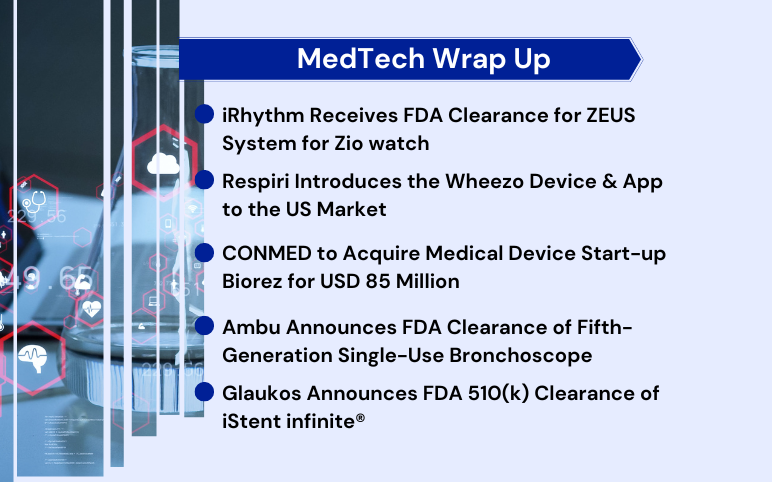
FDA Clearance to iRhythm’s ZEUS System; Respiri Introduces the Wheezo Device & App; CONMED to Acquire Biorez for $ 85 Million; FDA Clearance to Ambu’s Single-Use Bronchoscope; FDA 510(k) Clearance to Glaukos’s iStent infinite
iRhythm receives FDA clearance for ZEUS System for Zio watch iRhythm Technologies, a leading digital healthcare solutions firm focused on advancing cardiac care, announced that it received FDA 510(k) clearance for the ZEUS (Zio ECG Utilization Software) System for the Zio Watch. It is produced in partnership wit...
Read More...
Dec 12, 2019
Omega’s sixth funding round; Correvio’s drug failure; and BIMA acquisition
A leading global international investment firm, Omega Funds has announced the closure of its sixth fundraising USD 438 Million. More than decade old Life Sciences-based investment firm specialized in direct secondary transactions, Omega funds focuses in investing in Life Sciences vertical, aiming to fulfil the ...
Read More...
May 10, 2016
Anticoagulants: Market Analysis and Drug Sales Projections (VTE & AF)
According to DelveInsight researchers, the Anticoagulants (VTE & AF) Therapeutics Market was around $11.4 billion in 2015 and is estimated to reach $17.2 billion by 2020 growing at a CAGR of 8.59% from 2015 to 2020. DelveInsight Report “Anticoagulants-Market Insights & Drugs Sales Forecast (VTE & AF) -2...
Read More...
Apr 21, 2015
A Glimpse of the Anticoagulants Therapy Market; Major Players
VTE therapeutics & Atrial Fibrillation market was worth USD 4 billion in 2014 and USD 7.86 billion respectively. The VTE therapeutic market is expected to reach USD 5.5 billion by 2018 with a Compound Annual Growth Rate (CAGR) of 8.29% and Atrial Fibrillation market will reach on its peak value USD 13.28 billi...
Read More...








-Agonist.png)

