Balloon Catheters
Apr 03, 2025
Abbott Secures CE Mark for Volt™ Pulsed Field Ablation System and Unveils TRILUMINATE™ Trial Data on TriClip™ for Tricuspid Valve Repair; Zimmer Biomet’s RibFix Advantage® Fixation System Earns CE Mark; Teleflex Announces Initial Clinical Data from IDE Study Evaluating Ringer™ PBC in Coronary Perforation Treatment; GE HealthCare Launches Revolution™ Vibe CT, Bringing Unlimited One-Beat Cardiac Imaging and AI Solutions; TELA Bio Launches Larger OviTex® PRS Sizes in the U.S.
Abbott Received CE Mark for Volt™ Pulsed Field Ablation System, Offering New Therapy Option for Heart Rhythm Disorders On March 27, 2025, Abbott announced that it received the CE Mark in Europe for its VoltTM Pulsed Field Ablation (PFA) System, designed to treat patients with atrial fibrillation (AFib). Th...
Read More...
May 23, 2024
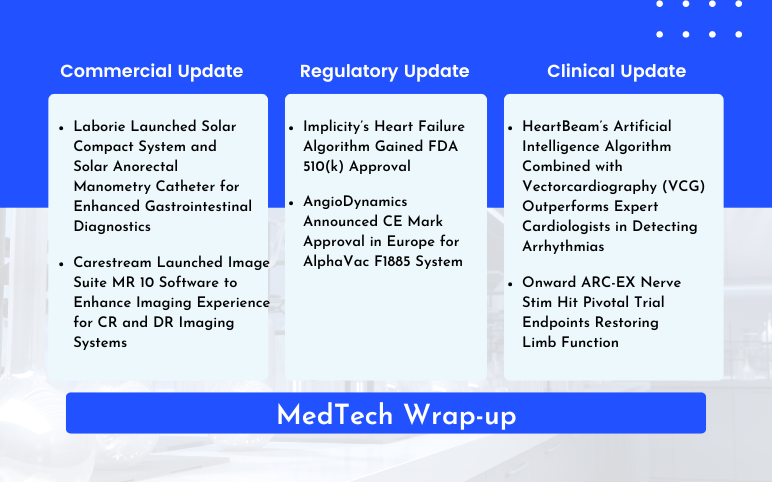
Laborie’s Enhanced Gastrointestinal Diagnostics; Carestream’s Image Suite MR 10 Software Launch; Implicity’s Heart Failure Algorithm FDA 510(k) Approval; AngioDynamics’ AlphaVac F1885 System CE Mark Approval; HeartBeam’s Artificial Intelligence Algorithm for Arrhythmias; Onward ARC-EX Nerve Stim Hit Pivotal Trial
Laborie Launched Solar Compact System and Solar Anorectal Manometry Catheter for Enhanced Gastrointestinal Diagnostics On May 20, 2024, Laborie Medical Technologies Corp., launched the Solar Compact System and Solar Anorectal Manometry Catheter, the first disposable HRAM catheter on the market. These devices sig...
Read More...
Apr 11, 2024
Carl Zeiss Meditec AG’s Acquisition of Dutch Ophthalmic Research Center; Johnson & Johnson’s Shockwave Medical Acquisition; Biora Therapeutics’ BT-600 Positive Results; Medtronic’s Single-Shot Mapping Ablation Catheter Positive Data; Abbott’s TriClip FDA Approval; Abbott’s Whole Blood Rapid Test FDA Clearance
Carl Zeiss Meditec AG Completed Acquisition of Dutch Ophthalmic Research Center (D.O.R.C); Companies Unite to Shape Ophthalmology Market On April 04, 2024, Carl Zeiss Meditec AG announced that it acquired D.O.R.C. (Dutch Ophthalmic Research Center) from the investment firm Eurazeo SE, Paris, France. T...
Read More...
Jan 04, 2024
HR Pharmaceuticals Announced Collaboration with Poiesis Medical; Enovis Acquired Lima Corporate; AnX Robotica Announced FDA Clearance for ProScan; FDA Breakthrough Device Designation for CGBio’s ‘NOVOSIS PUTTY’; EndoSound Received 510(k) Clearance for Breakthrough EVS Innovation; Boston Scientific Initiated AVANT GUARD Clinical Trial
HR Pharmaceuticals, Inc. Received Exclusive Commercialization Rights to Poiesis Medical’s Dual Balloon Catheter Technology in North America On December 28, 2023, HR Pharmaceuticals entered into a collaboration with Poiesis Medical LLC, to license Poiesis's Dual Balloon Catheter (Duette™). According to the terms ...
Read More...
Jun 22, 2023
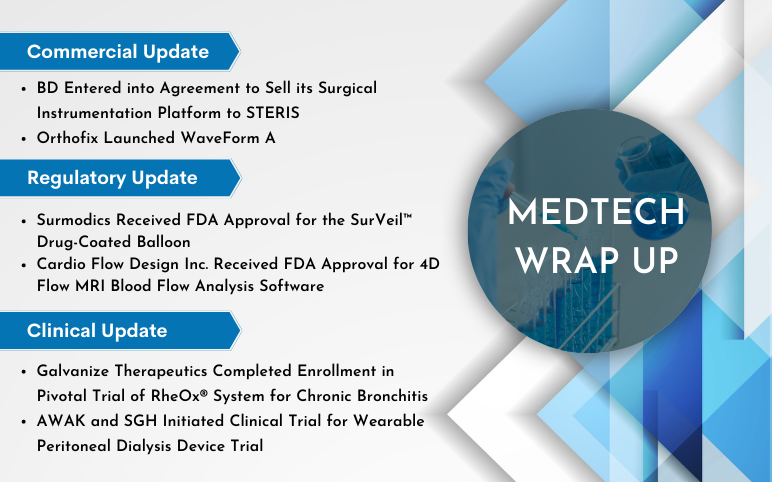
Orthofix Launched WaveForm A; BD Entered into Agreement with STERIS; Surmodics’s SurVeil™ Drug-Coated Balloon; Cardio Flow Design’s 4D Flow MRI Blood Flow Analysis Software; Galvanize Therapeutics’s RheOx® System; AWAK and SGH’s Wearable Peritoneal Dialysis Device Trial
Orthofix Launched WaveForm A – 3D Printed Anterior Lumbar Interbody On June 19, 2023, Orthofix Medical Inc., a leading global spine and orthopedics company, launched WaveForm® A, an interbody for Anterior Lumbar Interbody Fusion (ALIF) procedures. For the treatment of patients who require fusion due to degenerat...
Read More...
Jun 01, 2023
THINK Surgical’s TMINI Miniature Robotic System; Cepheid’s Xpert NPM1 Mutation Test; Anika’s Hyalofast® US Pivotal Phase III Study; EMVision’s Portable Brain Scanner Trial; HeartBeam and Samsung’s Strategic Alliance Agreement; Eosolutions’s Dr. Banner Balloon Guide Catheter
TMINI Miniature Robotic System by THINK Surgical Received FDA 510(k) Clearance On May 30, 2023, THINK Surgical, Inc., an innovator in the field of orthopedic surgical robots, received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its TMINI™ Miniature Robotic System. A wireless r...
Read More...
May 25, 2023
Eosolutions Corp’s Dr. Banner Balloon Guide Catheter; US FDA Approves the Cyltezo® Pen; Orlucent’s Handheld Mole Imaging System; Grünenthal’s Resiniferatoxin for Pain Associated with Knee Osteoarthritis; Element Science’s Jewel Patch Wearable Cardioverter Defibrillator; A.Menarini Diagnostics’s PRIME MDx Platform
Eosolutions Announces The Full Commercial Launch of The Dr. Banner Balloon Guide Catheter EOSolutions Corp., a forerunner in medical technology focused on offering high-quality catheter solutions, is pleased to introduce the Dr. Banner, Balloon Guide Catheter (BGC). Dr. Banner, developed in collaboration with In...
Read More...
May 04, 2023
Apyx Medical Received FDA 510(k) Clearance for the Use of Renuvion; Medtronic’s Next-generation Micra Leadless Pacing Systems; Concept Medical’s MagicTouch Sirolimus Coated Balloon Catheter; Vivasure’s Pivotal PATCH Study; SunMed Acquires the Vyaire’s Respiratory & Anesthesia Consumables Business; Eitan Medical’s Connected Avoset™ Infusion Platform
Apyx Medical Corporation Received FDA 510(k) Clearance for the Use of Renuvion® for Coagulation of Subcutaneous Soft Tissues Following Liposuction for Aesthetic Body Contouring On April 28, 2023, Apyx Medical Corporation, the manufacturer of a proprietary helium plasma and radiofrequency technology marketed and ...
Read More...
Feb 09, 2023
LivaNova Launched SenTiva DUO; Candela Launched Matrix System; 3M Launched Medical Adhesive; MONARCH Platform Performed First Kidney Stones Removal Procedure; Biosense Webster Presented Data of HELIOSTAR Balloon Ablation Catheter; FDA 510(k) Clearance to Aspivix’s Cervical Stabilizer Carevix
LivaNova Launched SenTiva DUO, an Implantable Pulse Generator On February 3, 2023, LivaNova PLC, a market-leading medical technology and innovation company launched SenTiva DUO™, an implantable pulse generator (IPG) with a dual-pin header that delivers VNS Therapy™ for the treatment of drug-resista...
Read More...
Oct 20, 2022
Biosense Webster’s HELIOSTAR Radiofrequency Balloon Ablation Catheter; Philips’s ClarifEye Augmented Reality Surgical Navigation Solution; NeuroLogica’s Elite Mobile Computed Tomography Devices; Medtronic’s Natural Conduction System for Heart; Pfizer & BioNTech’s Omicron BA.4/BA.5-Adapted Bivalent Booster Trial; Castle Biosciences’s TissueCypher® Barrett’s Esophagus Test
HELIOSTAR™ Radiofrequency Balloon Ablation Catheter Launched by Biosense Webster in Europe On October 12, 2022, Biosense Webster, a part of Johnson & Johnson Medical technology, announced the launch of HELIOSTAR™ Balloon Ablation Catheter, the first radiofrequency balloon ablation catheter in E...
Read More...







-Agonist.png)

