Biosimilar
Dec 01, 2023
Could Amgen’s Biosimilar Wezlana Pose a Challenge to Johnson & Johnson’s Stelara
The FDA recently approved Amgen’s Wezlana (ustekinumab-auub) for various inflammatory conditions, marking the first biosimilar approval referencing the popular J&J drug Stelara (ustekinumab). Wezlana, mirroring its reference product, is approved for treating multiple inflammatory diseases in adults, including m...
Read More...
Dec 15, 2021
Insights into some of the Biosimilar Drugs that are approved and launched in 2021
Since the first biosimilar drug was approved in 2006, the EU has led the way in biosimilar regulation. Over the last decade, the EU has authorized the most biosimilars globally, gathering significant expertise with their usage and safety. Over ten years of clinical experience has shown that biosimilars licensed by ...
Read More...
Nov 22, 2019
The long journey of Biosimilars: Is it an emerging opportunity?
Biologics are not new drugs, their use as a medicine has been around for over a century. The first use of Biologic drug dates back to the year 1796 when the vaccine to treat smallpox was created.They have been revolutionizing the treatment approaches for the past many years. Hormones, insulin, blood products, cytok...
Read More...
Mar 12, 2019
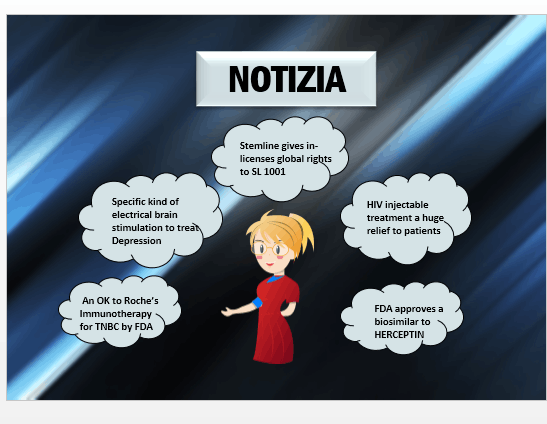
Notizia
Stemline gives in-licenses global rights to SL-1001 Stemline Therapeutics, Inc. is a biopharmaceutical company focused on development and commercialization of novel oncology therapeutics, has licensed worldwide rights to a preclinical molecule RET (rearranged during transfection) kinase inhibitor. The RET kinase...
Read More...
Sep 18, 2018
Biologic Parallel – A Perky Future Ahead
A biosimilar (copycat version) is a pharmaceutical product that is close, but not identical, copy of an approved biologic branded product. It is known as a biosimilar, rather than a biogeneric, because current technology does not approve of making an exact copy of a large molecule drug and the sky reaching cost of p...
Read More...
Nov 21, 2017
EMA to relocate to Amsterdam; Roche’s prospects; Amgen’s Humira Biosimilar delayed; Purdue’s opioid lawsuit
EMA to relocate to Amsterdam by March 2019 Results of the Monday's vote for the European Medicines Agency relocation led to the emergence of Amsterdam as the new headquarters, beating the other finalists Milan and Copenhagen. Nineteen European Union member states submitted proposals for the EMA headquarters, and Mil...
Read More...
Jan 13, 2017
Biosimilars: A benchmark in Pharmaceutical Business
The high cost of pharmaceuticals, especially Biologics, has been proven as an issue in the battle to control healthcare costs. The Biologics Price Competition and the Innovation Act has put forward the generic competition of Biologics or what is popularly called the Biosimilars. Biosimilars have been available in th...
Read More...
Oct 28, 2016
Biosimilar Market in India
Today's opportunity for Indian companies in biosimilars is not much different from that of the generics industry in 1984. According to Ministry of Commerce and Industry, India’s pharmaceutical export segment has more than doubled from $ 7.8 billion in 2008 to $ 16.5 billion in 2014. With biologic treatments int...
Read More...
Aug 19, 2016
Business Cocktail
Zymeworks licenses new drug discovery platform from Innovative Targeting Solutions Zymeworks, a biotech company working on bispecific antibodies, has signed a licensing deal with Innovative Targeting Solutions (ITS) for HuTARG, which works as a protein engineering platform, and is the first fully mammalian tech that...
Read More...





-Agonist.png)

