Biosimilar Market
Dec 01, 2023
Could Amgen’s Biosimilar Wezlana Pose a Challenge to Johnson & Johnson’s Stelara
The FDA recently approved Amgen’s Wezlana (ustekinumab-auub) for various inflammatory conditions, marking the first biosimilar approval referencing the popular J&J drug Stelara (ustekinumab). Wezlana, mirroring its reference product, is approved for treating multiple inflammatory diseases in adults, including m...
Read More...
Mar 01, 2022
GSK’s Covifenz; Idorsia’s Quviviq; GSK’s ZEJULA; EMA Expands its Nod for BMS, Eli Lilly, and Novartis Drugs; Cantex Secures Global Licence to Develop Azeliragon; Biocon Acquires Viatris’ Biosimilar Business
GSK’s Covifenz, A Plant-Based COVID Vaccine, Receives First Approval The recombinant COVID-19 vaccine developed by Medicago, now known as Covifenz, has received approval in Canada, the company's home country. Covifenz employs Coronavirus-Like Particle (CoVLP) technology, with the vaccine consisting of recombinan...
Read More...
Feb 24, 2022
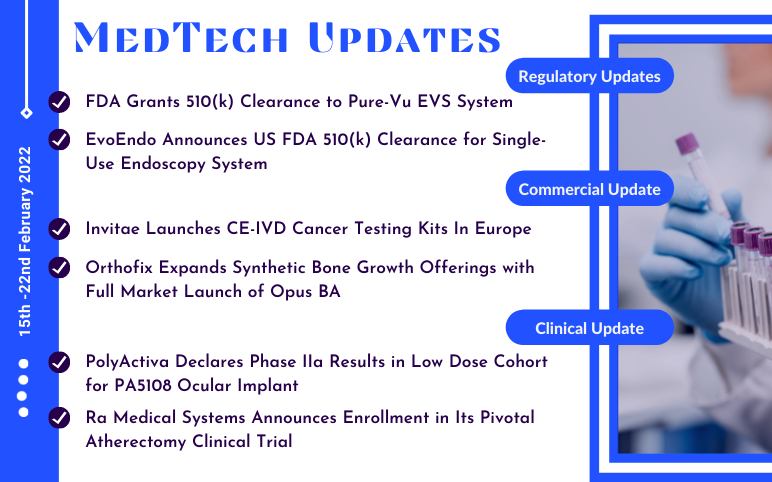
Motus GI’s Pure-Vu EVS System; EvoEndo’s Single-Use Unsedated TNE System; Orthofix’s OpusTM BA; Invitae’s CE-IVD Cancer Testing Kits; PolyActiva’s PA5108 Ocular Implant; Ra Medical’s DABRA excimer laser system
US FDA grants 510(k) clearance to Motus GI’s Pure-Vu EVS System On February 15, 2022, The Pure-Vu® EVS System received 510(k) clearance from the US Food and Drug Administration, according to Motus GI Holdings, Inc., a medical technology company that provides endoscopy solutions that improve clinical outcomes and...
Read More...
Feb 01, 2022
Immunocore’s Kimmtrak; Samsung Acquires Biogen’s Biosimilar Unit; Novavax’s COVID-19 Vaccine; CHMP Approves Dupixent (dupilumab); Glenmark’s High Blood Pressure Treatment; Roche’s Faricimab; GBT’s Voxelotor
Immunocore Receives FDA Approval for the First Uveal Melanoma Treatment, As Well As the First T-cell Receptor Therapeutics Uveal Melanoma, an aggressive eye cancer, has proven to be a tough nut to crack for researchers seeking a cure. However, with the approval of a new treatment, those with the disorder will ha...
Read More...
Dec 22, 2021
Analyzing the Key Trends Driving the Biosimilar Market Growth
The Biosimilars market is growing at a breakneck pace. The biosimilar market provides a compelling economic value proposition. This has been a considerable element in expanding the biosimilar market, and the future of this sector appears promising. Many pharmaceutical companies from across the globe are proactively...
Read More...
Dec 15, 2021
Insights into some of the Biosimilar Drugs that are approved and launched in 2021
Since the first biosimilar drug was approved in 2006, the EU has led the way in biosimilar regulation. Over the last decade, the EU has authorized the most biosimilars globally, gathering significant expertise with their usage and safety. Over ten years of clinical experience has shown that biosimilars licensed by ...
Read More...
Dec 03, 2021
Analyzing the Growth of the Biosimilar Market Through Years
The expiration of many biologic drugs patents has sparked biosimilar development and boosted the biosimilar market growth. Biosimilars are alternative versions of innovator biologic drugs. These are biological products that are similar replicas of the biopharmaceuticals that inspired them. Biosimilars are sometimes...
Read More...
Nov 22, 2019
The long journey of Biosimilars: Is it an emerging opportunity?
Biologics are not new drugs, their use as a medicine has been around for over a century. The first use of Biologic drug dates back to the year 1796 when the vaccine to treat smallpox was created.They have been revolutionizing the treatment approaches for the past many years. Hormones, insulin, blood products, cytok...
Read More...






-Agonist.png)

