Biosimilars
Dec 01, 2023
Could Amgen’s Biosimilar Wezlana Pose a Challenge to Johnson & Johnson’s Stelara
The FDA recently approved Amgen’s Wezlana (ustekinumab-auub) for various inflammatory conditions, marking the first biosimilar approval referencing the popular J&J drug Stelara (ustekinumab). Wezlana, mirroring its reference product, is approved for treating multiple inflammatory diseases in adults, including m...
Read More...
Aug 30, 2022
Aktis’s Novel Targeted Alpha Radiopharmaceuticals; Koye Partners with Sonde Health; Novartis to Spin Off Sandoz Business; Alcon to Buy Aerie Pharma; Fast Track Designation to Merck’s MK-2060; FDA Approves Ibrutinib for Chronic GvHD; French Authorities Clears BrainVectis’s Clinical Trial; Takeda’s Dengue Vaccine TAK-003 Gets Approval in Indonesia
Aktis Oncology Raises USD 84 Million To Advance Novel Targeted Alpha Radiopharmaceuticals Aktis Oncology has raised an additional USD 84 million in its Series A round, adding to the USD 72 million raised last year to help bring its radiopharmaceuticals to market. The extension to the first round included Merck's...
Read More...
Feb 24, 2022
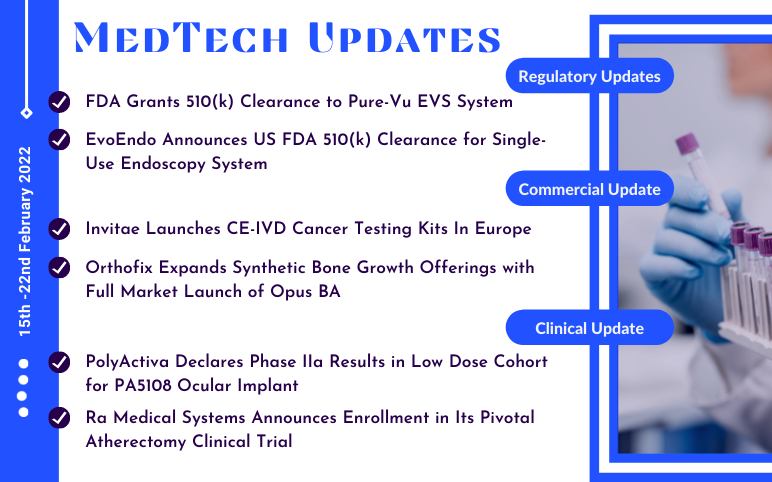
Motus GI’s Pure-Vu EVS System; EvoEndo’s Single-Use Unsedated TNE System; Orthofix’s OpusTM BA; Invitae’s CE-IVD Cancer Testing Kits; PolyActiva’s PA5108 Ocular Implant; Ra Medical’s DABRA excimer laser system
US FDA grants 510(k) clearance to Motus GI’s Pure-Vu EVS System On February 15, 2022, The Pure-Vu® EVS System received 510(k) clearance from the US Food and Drug Administration, according to Motus GI Holdings, Inc., a medical technology company that provides endoscopy solutions that improve clinical outcomes and...
Read More...
Feb 22, 2022
Sandoz’s Generic Revlimid; Agios’ Pyrukynd; Organon Announces 4Q & Full-year Earnings Report; BMS’ CAR-T Drug Breyanzi; Sanofi & Regeneron’s Dupixent Trial; AZ & Daiichi’s Drug Enhertu; Bayer’s Drug Kerendia; Lilly Releases Mirikizumab Data
Sandoz Launches generic Revlimid in 19 European Countries, Bringing a Flood of Competition to BMS' Megablockbuster Since Bristol Myers Squibb acquired Celgene and its megablockbuster Revlimid, the company has been bracing for the day when the multiple myeloma superstar would face generic competition. Sandoz, a s...
Read More...
Dec 22, 2021
Analyzing the Key Trends Driving the Biosimilar Market Growth
The Biosimilars market is growing at a breakneck pace. The biosimilar market provides a compelling economic value proposition. This has been a considerable element in expanding the biosimilar market, and the future of this sector appears promising. Many pharmaceutical companies from across the globe are proactively...
Read More...
Dec 03, 2021
Analyzing the Growth of the Biosimilar Market Through Years
The expiration of many biologic drugs patents has sparked biosimilar development and boosted the biosimilar market growth. Biosimilars are alternative versions of innovator biologic drugs. These are biological products that are similar replicas of the biopharmaceuticals that inspired them. Biosimilars are sometimes...
Read More...
Nov 07, 2017
Teva sells generics; Valeant trades; Roche’s Genentech; J&J’s next potential frontier
Teva looks to sell generics in China through joint venture with Guangzhou Pharma As the world’s largest generics maker, Teva still doesn’t have much of a presence in China, even though it is a country relies heavily on generics. The Israeli company is looking to remedy that as Teva has said it “has no agreement with...
Read More...
Apr 27, 2017
Merck sells biosimilars; AbbVie’s PARP; Pharma heads; Biogen looks to M&A
Merck sells biosimilars business to Fresenius Merck has divested its biosimilars business to fellow German drug maker Fresenius in a deal potentially worth 670 million euros. The drug maker said the move is aligned with a strategy of focusing on innovative medicines while enabling it to exploit its biosimilars portf...
Read More...
Jul 04, 2014
EMA Biosimilars Approvals
In the European Union (EU), a legal framework for approving biosimilars was established in 2003. This framework means that biosimilars can only be approved centrally via the European Medicines Agency (EMA) and not nationally. On 27 June 2014, the European Medicines Agency’s (EMA’s) Committee for Medicinal Produc...
Read More...







-Agonist.png)

