Boehringer Ingelheim
Feb 27, 2024
Survodutide Phase II trial Shows Groundbreaking Results in Liver Disease; GSK Announces Positive Headline Results from EAGLE-1 Phase III Trial; Dupixent sBLA Accepted for FDA Priority Review; Biogen’s QALSODY Received Positive Opinion from CHMP; FDA Granted Orphan Drug Designation to Immune-Onc’s IO-202; Artiva Biotherapeutics’s AlloNK® in Lupus Nephritis
Survodutide Phase II trial Shows Groundbreaking Results in Liver Disease due to MASH, with Significant Improvements in Fibrosis Boehringer Ingelheim has reported that in a Phase II trial, a significant proportion of adults treated with survodutide (BI 456906), up to 83.0%, showed a notable enhancement in metabol...
Read More...
Oct 10, 2023
Amgen to Acquire Horizon Therapeutics; Sanofi and Teva Announce Collaboration; Boehringer Obesity Drug Trial Update; FDA Places Partial Clinical Hold on IND for Lacutamab in CTCL/PTCL; Anaptys Announces Phase 3 Clinical Trial Results of Imsidolimab; Orphan Drug Designation to GC Biopharma’s GC1126A
Sanofi and Teva Announce Exclusive Collaboration to Deliver Inflammatory Bowel Disease Treatment Sanofi and Teva Pharmaceuticals, a subsidiary of Teva Pharmaceutical Industries Ltd. in the United States, have announced a collaboration to co-develop and co-commercialize asset TEV'574, which is currently in Phase ...
Read More...
Jun 27, 2023
FDA Approves Jardiance for Type 2 Diabetes; FDA Approves Pfizer’s LITFULO for Alopecia Areata; Sarepta Therapeutics’s ELEVIDYS Approval; Tonix Pharmaceuticals to Acquire Two Migraine Products from Upsher-Smith; FibroGen’s Phase 3 ZEPHYRUS-1 Study of Pamrevlumab; FDA Orphan Drug Designation to ERAS-801 for Malignant Glioma
FDA Approves Jardiance for the Treatment of Type 2 Diabetes in Children 10 Years and Older Boehringer Ingelheim and Eli Lilly and Company announced that the FDA has approved Jardiance® (empagliflozin) 10 mg and 25 mg tablets to decrease blood sugar together with diet and exercise in children 10 years and older w...
Read More...
Aug 10, 2020
Boehringer Ingelheim; Roche demonstrated new analyses of their drugs in patients with ILDs during the American Thoracic Society (ATS) Virtual conference
ATS 2020 Virtual is scheduled to be held from August 5 to August 10, and it is exciting to watch the key presentations by Pharmaceutical Companies specifically focused on pulmonary disease, critical illness, and sleep disorders. Boehringer Ingelheim presented new analyses of Ofev data in patients with chronic fi...
Read More...
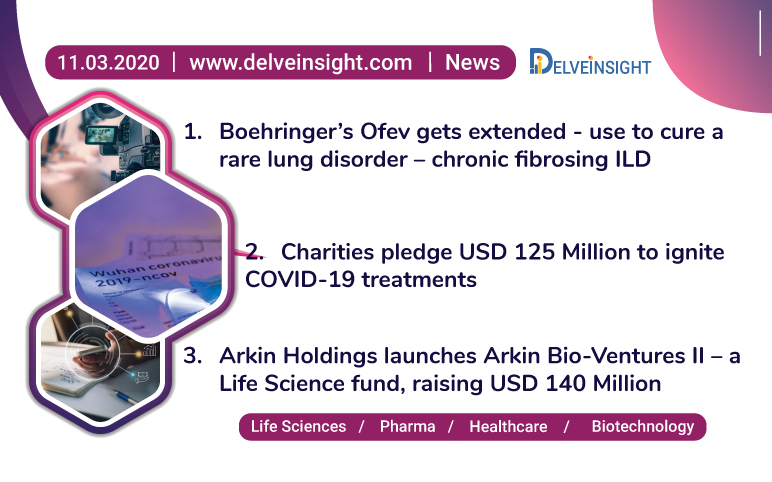
Mar 11, 2020
Ofev’s expanded use; Arkin Bio-Ventures II launch; USD 125 M for COVID-19 treatment
Boehringer Ingelheim’s Ofev (nintedanib) has received FDA recommendation for the treatment of chronic fibrosing interstitial lung diseases (ILD) with a progressive phenotype Interstitial Lung Disease - ILD – a group of a large number of lung disorders resulting in scarring or fibrosis of lungs, caused by conditi...
Read More...
Jan 14, 2020
Eli Lilly to acquire Dermira, Boehringer buys Enleofen’s IL-11 platform, Biogen acquires Pfizer’s Alzheimer’s drug
Eli Lilly has announced to buy a biopharmaceutical company, Dermira, for a whopping sum of USD 1.1 Billion in an all-cash transaction. Through the acquisition, Eli Lilly is planning to expand its immunology pipeline by adding lebrikizumab, which is a novel, investigational, monoclonal antibody. The drug is desi...
Read More...
Sep 23, 2019
Boehringer Ingelheim ties up with Inflammasome Therapeutics
Boehringer Ingelheim and Inflammasome Therapeutics Inc. have announced that they have entered into a definitive a co-development and license agreement to develop therapies for the patients with retinal diseases.The deal is about jointly developing three novel therapies for retinal diseases with the help of Inflamma...
Read More...
Sep 09, 2019
FDA approves Ofev for interstitial lung disease
Recently, the US FDA has approved a new drug therapy to treat adults with interstitial lung disease associated with systemic sclerosis or scleroderma, called SSc-ILD. Ofev (nintedanib) capsules, the drug developed by Boehringer Ingelheim Pharmaceuticals, has shown efficacy in slowing down the rate of decline in pul...
Read More...
Nov 10, 2017
Business Cocktail
Takeda and Portal Instruments partner to bring about needle-free form of drug delivery Takeda Pharmaceutical and Portal Instruments have partnered with a deal worth $100 million to develop a needle-free drug delivery device and increase patient compliance rates for biologic medicines manufactured by the former compa...
Read More...
Feb 10, 2017
Chronic Kidney Disease: Complex Debilitating Condition
Chronic Kidney Disease (CKD) is a major global public health problem which progresses slowly and get worse over time. It is the 8th leading cause of death in United States, and is characterized by kidney damage and reduced kidney function. According to National Kidney Foundation, approximately 26 millions of adults ...
Read More...







-Agonist.png)

