Cardiac Ablation Devices Market
Jan 11, 2024
Medtronic Announced World’s First Approval for MiniMed 780G System; CE Mark for Medtronic’s Next Generation Micra Leadless Pacing Systems; Biosense Webster Received Approval for VARIPULSE PFA Platform; Rivermark Medical Updated on Flostent System; Cala Disclosed TAPS Therapy Data for Essential Tremor; Medline Introduced Transparent Wound Dressing
Medtronic Received CE Mark for its Next Generation Micra Leadless Pacing Systems On January 5, 2024, Medtronic announced that the Micra AV2 and Micra VR2, the next generation of its industry-leading tiny, leadless pacemakers, earned the CE (Conformité Européenne) Mark. The world's tiniest pacemakers, the rMic...
Read More...
Nov 17, 2022
Medtronic Launches Infusion Set for Insulin Pumps; Penumbra’s Virtual Reality-Based Rehabilitation System; FDA Approval to Genesis’s Chocolate Touch® Drug-coated Balloon PTA Catheter; FDA Nod to AEYE’s AI-based Autonomous Screening; Alpheus Medical Treats First High-Grade Glioma Brain Cancer Patient with its Proprietary Platform; Karidum’s Next Generation Globe® Pulsed Field System
Medtronic Launches World's First and Only Infusion Set for Insulin Pumps that Doubles Wear Time up to 7 days in the US On November 15, 2022, Medtronic plc, a global leader in healthcare technology, announced the US launch of the Medtronic Extended infusion set, the first and only infusion set labeled for up to ...
Read More...
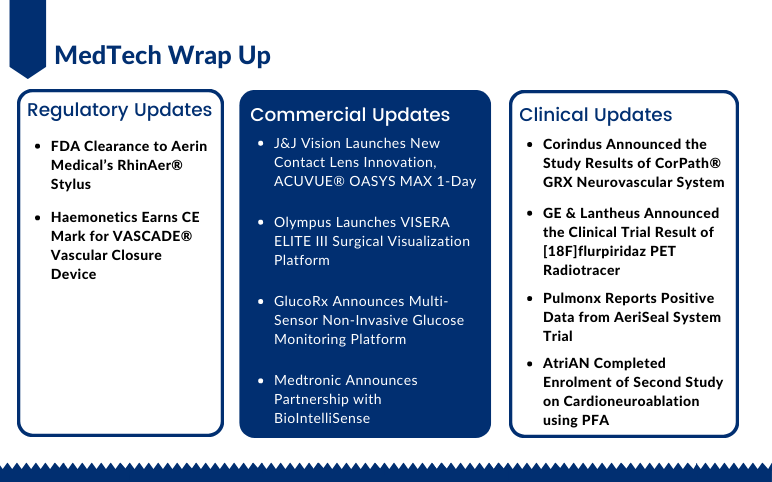
Sep 15, 2022
Aerin Medical’s RhinAer Stylus; CE Mark for Haemonetics’s VASCADE Vascular Closure Device; J&J Vision Launches ACUVUE OASYS MAX 1-Day; Olympus Launches VISERA ELITE III Surgical Visualization Platform; GlucoRx’s Non-Invasive Glucose Monitoring Platform; Medtronic-BioIntelliSense’s Partnership; Corindus’s CorPath GRX Neurovascular System Trial; GE & Lantheus [18F]flurpiridaz PET Radiotracer Trial; Pulmonx’s AeriSeal System Trial; AtriAN’s Second Study on Cardioneuroablation
Johnson & Johnson Vision Launches New Contact Lens Innovation ACUVUE® OASYS MAX 1-Day for Meeting the Needs of Digitally Intense Lifestyles On September 12, 2022, Johnson & Johnson Vision, a part of Johnson & Johnson and a global leader in the eyecare market, had announced the launch of its newest in...
Read More...
Sep 01, 2022
Miracor Medical’s Picso Pivotal Study; Medtronic’s Extravascular ICD; Galaxy Medical’s CENTAURI Pulsed Electric Field System; UltraSight’s Novel Cardiac AI Technology; Oticon Launched the World’s Smallest Hearing Aid; Medtronic Completes Acquisition of Affera
FDA Approved IDE for Picso® Pivotal Study of Miracor Medical On August 23, 2022, Miracor Medical SA, a provider of innovative solutions for the treatment of severe cardiac diseases, announced that the FDA has approved an Investigational Device Exemption (IDE), allowing the company to begin a pivotal study with i...
Read More...
Aug 25, 2022
Edwards’s Pascal Precision System; Abbott’s New Spinal Cord Stimulation Device; Imagin to Acquire enCAGE Coil Precision Ablation System; Boston Heart Diagnostics Launches LipidSeqTM; Rocket VR Health Partners with Penn Medicine’s Abramson Cancer Center; Thermedical’s Clinical Trial to Treat Ventricular Tachycardia
Edwards Pascal Precision Transcatheter Mitral And Tricuspid Valve Repair System Receives CE Mark On August 17, 2022, Edwards Lifesciences Corporation, an American medical technology company headquartered in Irvine, California, specializes in artificial heart valves and hemodynamic monitoring. The company announc...
Read More...



-Agonist.png)

