Cardiovascular disease
Mar 12, 2024
BeiGene’s BRUKINSA Gets FDA Accelerated Approval; GSK’s Positive Results in DREAMM-8 Phase III; Sandoz’s Denosumab Biosimilars FDA Approved; Terns Pharma’s TERN-701 Receives Orphan Drug Designation; Wegovy® Approved in US for Overweight Cardiovascular Risk; Travere Therapeutics Submits sNDA for FILSPARI IgAN Full Approval
BeiGene Receives FDA Accelerated Approval for BRUKINSA in Relapsed/Refractory Follicular Lymphoma BeiGene, Ltd., has declared that the FDA has provided accelerated approval for BRUKINSA® (zanubrutinib) to be used in treating adult patients with relapsed or refractory (R/R) follicular lymphoma (FL), when used alo...
Read More...
Sep 13, 2023
Biosurgery: Advancing Medicine through Biologically Derived Solutions
In recent years, the healthcare and medical devices market has undergone a remarkable transformation, largely fueled by advancements in technology and growth in innovation. One of the major standout developments in the healthcare industry is the rise of the biosurgery market and the related product demand, whic...
Read More...
Oct 11, 2022
Merck’s Sotatercept Trial Result; PARP Rivals Closing in on AstraZeneca and Merck’s Lynparza; FDA Clears GSK’s Boostrix for Pertussis; Fast Track Designation to Nanoscope Therapeutics’s MCO-010; FDA Awards Fast Track Designation to Eftilagimod Alpha Plus Pembrolizumab; Solid Biosciences to Acquire AavantiBio
Merck’s Sotatercept Clears Phase III Trial Merck’s USD 11.5 billion acquisition of Acceleron last year was based on the promise of pulmonary arterial hypertension (PAH) candidate sotatercept, which has recently met the mark in a much-anticipated phase III trial. The STELLAR trial found that adding the activin re...
Read More...
Aug 02, 2022
Bristol-Myers Squibb’s Opdivo & Yervoy Combo Trial; Sarepta’s Gene Therapy SRP-9001 for DMD; Ionis’s End-Stage Renal Disease Drugs; FDA Approves Arcutis’s Zoryve Cream; Gilead’s Biktarvy for HIV and Hepatitis B; FDA to Review Biogen’s ALS Therapy Tofersen
Bristol-Myers Squibb’s Opdivo and Yervoy Combo Fails in Phase III Trial Bristol-Myers Squibb has reported that their Opdivo and Yervoy checkpoint inhibitor combo failed a phase III trial as adjuvant (post-surgery) therapy for renal cell carcinoma (RCC), the most frequent type of kidney cancer. The CheckMate -914...
Read More...
Jun 14, 2022
GSK’s RSV Vaccine Clears Phase III Test in Adults; Roche’s Tecentriq for Adjuvant NSCLC; Owkin Bags $ 180 million from BMS; EU Approves Roche’s Mosunetuzumab; Dostarlimab Elicits Clinical Complete Response in dMMR Rectal Cancer; FDA Backs Bluebird’s CALD Gene Therapy; Takeda’s Dengue Fever Vaccine TAK-003; FDA Approves Dupilumab
GSK Starts Preparations for Regulatory Filings as RSV Vaccine Clears Phase III Test in Adults GSK plans to initiate preparation for regulatory submissions for its respiratory syncytial virus (RSV) vaccine after the vaccine performed well in the much-anticipated AReSVi 006 trial in individuals aged 60 and above.&...
Read More...
Jun 07, 2022
CG Oncology Teams Up With Merck & BMS; Parse Biosciences Partners With Molecular Diagnostics Korea; Amgen’s Olpasiran; Elevar Therapeutics Announces Results of Rivoceranib; FDA Approves GSK’s Measles Vaccine; Sage & Biogen Reveals Postpartum Depression Trial Result
FDA Gives Green Signal to First Measles Vaccine in Almost 50 Years GSK announced that the company's vaccine for protection against mumps, measles, and rubella (MMR) had been approved by the U.S. FDA. According to data from six clinical studies, the company filed a Biologics License Application in August 2021. ...
Read More...
Jan 13, 2022
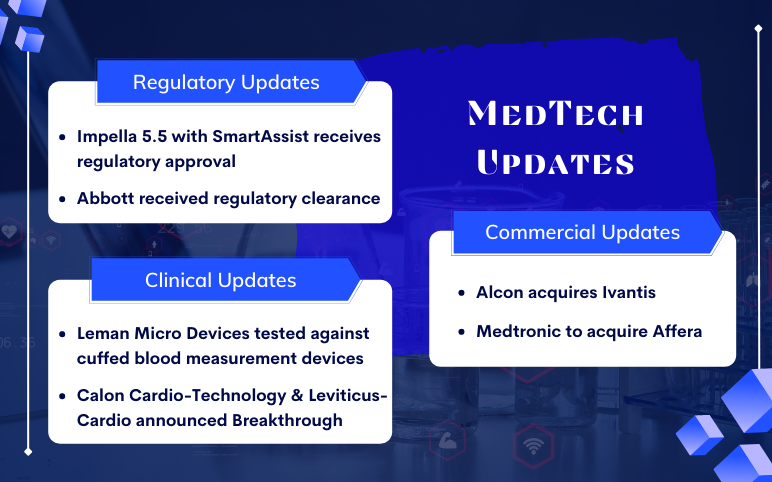
Leman Micro Devices’ e-Checkup system; Calon Cardio-Technology & Leviticus-Cardio announced Breakthrough; Alcon buys Ivantis; Medtronic to acquire Affera; Abiomed’s Impella 5.5 with SmartAssist; Abbott’s EnSite X EP System with EnSite OT
Leman Micro Devices tested against the cuffed blood measurement devices reported positive results On January 07, 2022, the e-Checkup system by Leman Micro Devices tested in the world’s first clinical trial against the cuffed blood measurement devices reported positive results. The e-Checkup and its sma...
Read More...
Jul 13, 2021
Bayer’s CKD Drug; Enochian’s RNA Hijack for HBV; Novo Nordisk’s to get Prothena’s ATTR Amyloidosis Business; J&J’s COVID-19 Vaccine Struggle
Bayer Scores FDA-Approval for its CKD Drug Finerenone Following a priority review, the USFDA finally gave its thumbs-up to Bayer AG’s Kerendia (finerenone) for chronic kidney disease (CKD) associated with type 2 diabetes (T2D) based on the results from the Phase III FIDELIO-DKD study. Finerenone is the first ...
Read More...
Jul 12, 2021
With advancements in Innovative Technology, Cardiac Monitoring Devices Market is Booming Significantly
Cardiac monitoring devices are important for cardiovascular care, to assess the occurrence and seriousness of cardiac failure and to evaluate the success of therapies such as medications, procedures, and device implants. Cardiovascular diseases (CVDs) are a global cause of death, which can be prevented. In most cas...
Read More...
Jun 22, 2021
Horizon/ Arrowhead Deal; Dr Reddy’s Lab’s Drug US Launch; Targovax’s ONCOS-102; RedHill’s Oral Opaganib in COVID-19
Horizon Dishes Out Million Dollars to get Arrowhead's RNAi Drug Arrowhead Pharmaceuticals and Horizon Therapeutics have announced a research and development collaboration to advance the treatment landscape for uncontrolled gout. Under the terms and conditions, Arrowhead is bound to receive an upfront sum of U...
Read More...








-Agonist.png)

