Chronic Kidney Disease
Feb 03, 2025
OZEMPIC’s New Approval Cements Novo’s Lead in GLP-1 Market
The recent approval of OZEMPIC reinforces the growing trend of GLP-1 drugs being used for conditions beyond diabetes and weight loss. Novo Nordisk’s blockbuster GLP-1 medication, OZEMPIC, has secured another label expansion, as the FDA has now approved it for treating chronic kidney disease. The approval for chr...
Read More...
Nov 12, 2024
AUCATZYL Approved for R/R B-ALL; FDA Accepts NDA for Unicycive’s Oxylanthanum Carbonate; AstraZeneca and Amgen Report Positive Results in Chronic Rhinosinusitis; Nipocalimab Granted Breakthrough Designation for Sjögren’s Disease; AbbVie’s Schizophrenia Drug Fails Phase Studies
FDA Approves Autolus's AUCATZYL for Relapsed/Refractory B-cell Acute Lymphoblastic Leukemia Autolus Therapeutics has achieved a significant milestone with FDA approval for AUCATZYL (obecabtagene autoleucel), a next-generation CAR T-cell therapy for adults with relapsed or refractory B-cell acute lymphoblastic le...
Read More...
Sep 27, 2024
Promising Data from the First Dedicated Kidney Outcomes Trial with GLP-1 Receptor Agonist, Semaglutide, in Patients with Type 2 Diabetes and Chronic Kidney Disease
On 11 September 2024, the latest results from the FLOW trial were presented at the European Association for the Study of Diabetes (EASD) conference in Madrid, Spain. The FLOW trial, initiated in 2019, was a global, multinational, randomized controlled trial conducted across 28 countries, 387 sites, and 3,533 partic...
Read More...
Sep 27, 2024
Pooled Analysis of Finerenone Presented at EASD 2024, Poised to Strengthen its Position in the Cardio-Kidney-Metabolic Market Further
Data from an integrated pooled analysis of finerenone across three Phase III trials involving heart failure, chronic kidney disease (CKD), and Type 2 diabetes (T2D) was presented at the 2024 European Association for the Study of Diabetes (EASD) Congress. This analysis was conducted with data from the FIDELIO-DKD2 a...
Read More...
Apr 02, 2024
AstraZeneca’s Voydeya FDA Approval; Akebia’s Vafseo FDA Approval; Bristol Myers Squibb’s Phase III YELLOWSTONE Trial Update; Astellas’ IZERVAY FDA Approval; AstraZeneca’s Truqap and Faslodex MHLW Approval
Voydeya Receives FDA Approval as Supplemental Treatment with Ravulizumab or Eculizumab for Managing Extravascular Hemolysis in Adult Patients with PNH Voydeya (danicopan) has received approval in the United States for use alongside ravulizumab or eculizumab in treating extravascular hemolysis (EVH) in adults dia...
Read More...
Mar 18, 2024
Promising Nephrotic Syndrome Treatments: A Look into the Future
Nephrotic syndrome is a clinical condition characterized by significant protein in the urine (exceeding 40 mg/m2/h), leading to low levels of albumin (below 30 g/L), which in turn causes high levels of lipids, swelling, and a range of associated complications. The epidemiological assessment of nephrotic syndrome...
Read More...
Mar 08, 2024
Breakthroughs in Alport Syndrome Treatment: A New Era of Hope
Alport syndrome, an inherited disease, predominantly manifests in its X-linked form, constituting around 80% of cases. Without intervention, roughly 90% of affected males face kidney failure by age 40, whereas females typically experience a slower progression to this condition. Many Alport syndrome cases go unnotic...
Read More...
Nov 30, 2023
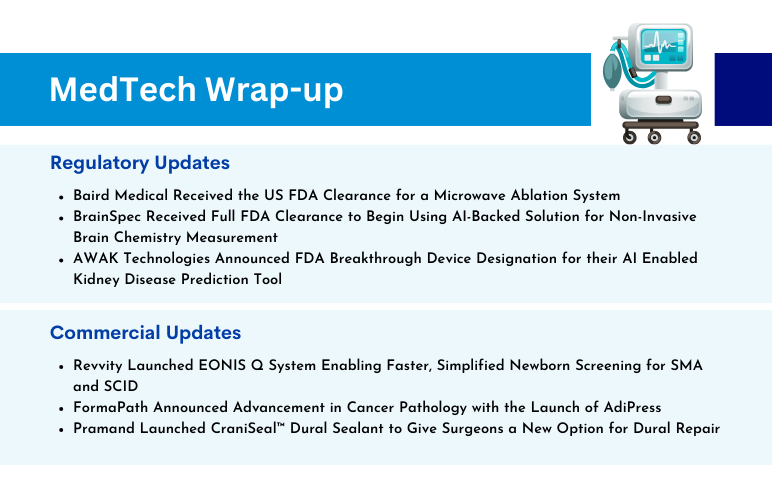
Baird Medical’s Microwave Ablation System; BrainSpec’s AI-Backed Solution for Non-Invasive Brain Chemistry Measurement; AWAK’s AI-Enabled Kidney Disease Prediction Tool; Revvity’s EONIS Q System; FormaPath Announced Advancement in Cancer Pathology; Pramand Launched CraniSeal Dural Sealant
Baird Medical Received the US FDA Clearance for a Microwave Ablation System On November 22, 2023, Baird Medical announced the US Food and Drug Administration clearance of the company’s microwave ablation (MWA) system and disposable needles for use in the US. The clearance allows the use of the technology to a...
Read More...
Nov 07, 2023
New Asundexian Phase III Study Result; Zibotentan/Dapagliflozin Combination Demonstrated Significant Albuminuria Reduction Chronic Kidney Disease; Orphan Drug Designation to Rhenium Obisbemeda; FDA Approves Merck’s KEYTRUDA Plus Gemcitabine and Cisplatin for Biliary Tract Cancer; Orphan Drug Designation to Ayala’s AL102; GARDP Announces Successful Phase 3 Trial of of Uncomplicated Gonorrhea
Positive Results Announced in Largest Pivotal Phase 3 Trial of a First-in-Class Oral Antibiotic to Treat Uncomplicated Gonorrhea The Global Antibiotic Research & Development Partnership (GARDP), in partnership with Innoviva, Inc. (Nasdaq: INVA), had announced a significant milestone. They revealed that zolif...
Read More...
Oct 23, 2023
Ardelyx Overcomes Hurdles to Secure FDA Approval for Xphozah in Chronic Kidney Disease Treatment
Ardelyx has struck gold on its third attempt with Xphozah (tenapanor), the chronic kidney disease medication. Following two prior rejections, the FDA has granted its long-awaited approval. Xphozah, an innovative phosphate absorption inhibitor, is now officially sanctioned for the management of serum phosphate level...
Read More...





-Agonist.png)

