chronic kidney disease market
Feb 03, 2025
OZEMPIC’s New Approval Cements Novo’s Lead in GLP-1 Market
The recent approval of OZEMPIC reinforces the growing trend of GLP-1 drugs being used for conditions beyond diabetes and weight loss. Novo Nordisk’s blockbuster GLP-1 medication, OZEMPIC, has secured another label expansion, as the FDA has now approved it for treating chronic kidney disease. The approval for chr...
Read More...
Nov 30, 2023
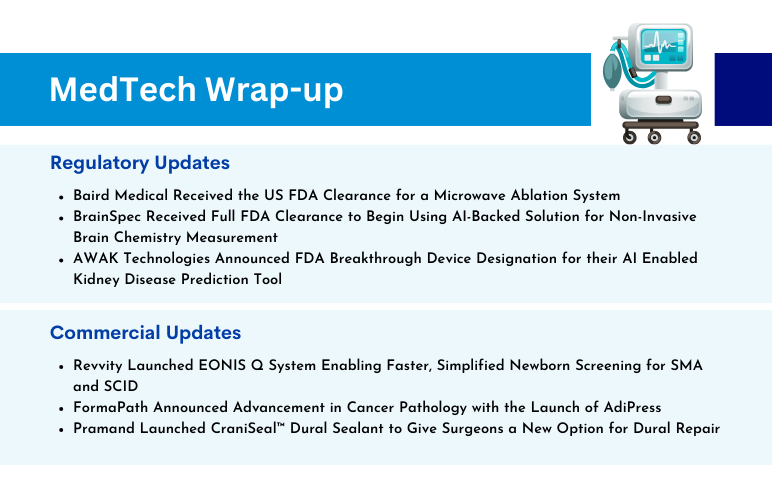
Baird Medical’s Microwave Ablation System; BrainSpec’s AI-Backed Solution for Non-Invasive Brain Chemistry Measurement; AWAK’s AI-Enabled Kidney Disease Prediction Tool; Revvity’s EONIS Q System; FormaPath Announced Advancement in Cancer Pathology; Pramand Launched CraniSeal Dural Sealant
Baird Medical Received the US FDA Clearance for a Microwave Ablation System On November 22, 2023, Baird Medical announced the US Food and Drug Administration clearance of the company’s microwave ablation (MWA) system and disposable needles for use in the US. The clearance allows the use of the technology to a...
Read More...
Oct 23, 2023
Ardelyx Overcomes Hurdles to Secure FDA Approval for Xphozah in Chronic Kidney Disease Treatment
Ardelyx has struck gold on its third attempt with Xphozah (tenapanor), the chronic kidney disease medication. Following two prior rejections, the FDA has granted its long-awaited approval. Xphozah, an innovative phosphate absorption inhibitor, is now officially sanctioned for the management of serum phosphate level...
Read More...
Oct 12, 2023
B. Braun’s Introcan Safety 2 IV Catheter; Everly Health’s At-Home Collection Kidney Health Test; FDA Clearance to Sanguina’s AnemoCheck Home; FDA Clearance for the DePuy’s TriALTIS™ Spine System; Biotronik’s Spinal Cord Stimulation Tech; Contego Medical’s Performance III Direct Transcarotid Access Stenting Trial
B. Braun Launched Introcan Safety® 2 IV Catheter with Multi-Access Blood Control Designed to Protect Clinicians Every Time the Hub is Accessed On October 11, 2023, B. Braun Medical Inc. (B. Braun), a leader in smart infusion therapy, announced the launch of its new Introcan Safety® 2 IV Catheter with Multi-Acces...
Read More...
Jun 12, 2023
Evolving Therapeutics in Chronic Kidney Disease (CKD) Treatment Market
Chronic kidney disease burden and diagnostic barriers Chronic kidney disease (CKD) is a progressive and irreversible ailment characterized by gradual loss of kidney function. It is clinically defined by a glomerular filtration rate of less than 60mL/min/1.73m2 or albuminuria of at least 30mg per 24 hours, or any...
Read More...
Dec 23, 2019
Autologous cell therapy market: A new paradigm for kidney diseases
The past few decades have seen significant advancements in the medical sector. Technological intervention is one of them. Discovery of the remarkable property of Stem cells to be able to differentiate into different types of cell forms, replicate and express themselves as their parents is one of the milestone-disco...
Read More...

-Agonist.png)

