Chronic Kidney Disease
Oct 12, 2023
B. Braun’s Introcan Safety 2 IV Catheter; Everly Health’s At-Home Collection Kidney Health Test; FDA Clearance to Sanguina’s AnemoCheck Home; FDA Clearance for the DePuy’s TriALTIS™ Spine System; Biotronik’s Spinal Cord Stimulation Tech; Contego Medical’s Performance III Direct Transcarotid Access Stenting Trial
B. Braun Launched Introcan Safety® 2 IV Catheter with Multi-Access Blood Control Designed to Protect Clinicians Every Time the Hub is Accessed On October 11, 2023, B. Braun Medical Inc. (B. Braun), a leader in smart infusion therapy, announced the launch of its new Introcan Safety® 2 IV Catheter with Multi-Acces...
Read More...
Jun 12, 2023
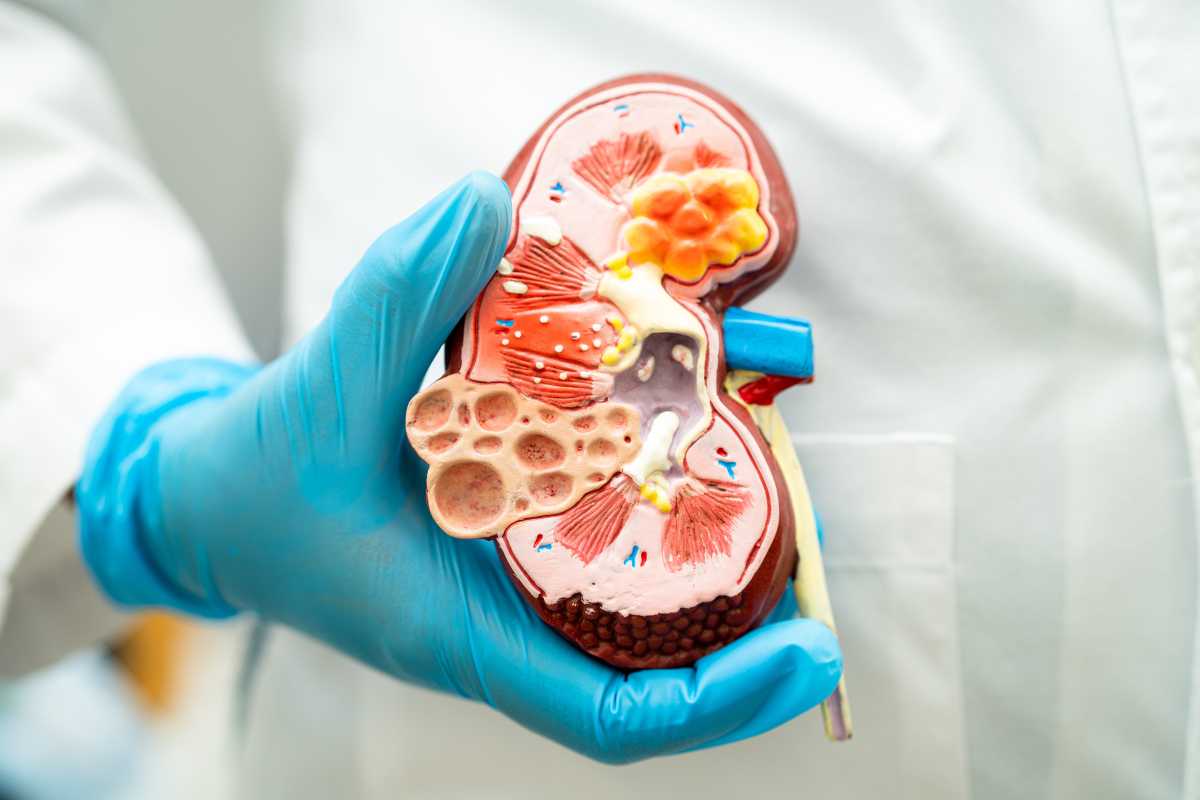
Evolving Therapeutics in Chronic Kidney Disease (CKD) Treatment Market
Chronic kidney disease burden and diagnostic barriers Chronic kidney disease (CKD) is a progressive and irreversible ailment characterized by gradual loss of kidney function. It is clinically defined by a glomerular filtration rate of less than 60mL/min/1.73m2 or albuminuria of at least 30mg per 24 hours, or any...
Read More...
Feb 07, 2023
Merck’s Keytruda Wins Another FDA Approval; Sanofi Pauses Trial of Myasthenia Gravis Drug, tolebrutinib; FDA Approves GlaxoSmithKline’s Jesduvroq; FDA IND Application Clearance for Hinova’s HP518; FDA Fast Track Designation to Endogena’s EA-2353; Amylyx Updates on Global Phase 3 PHOENIX Trial
Merck Wins Another FDA Approval for Blockbuster Keytruda Merck & Co arrived just two months after GSK celebrated a positive phase III result with its checkpoint inhibitor Jemperli as a first-line therapy for endometrial cancer. Keytruda (pembrolizumab) from Merck improved progression-free survival (PFS) vers...
Read More...
Sep 13, 2022
Amgen Announced the Result of its CodeBreak-200 Trial; FDA Clears Bristol-Myers Squibb’s Deucravacitinib; BioNTech’s Amplified CAR-T Therapy; TIL Therapy Improves on Yervoy in Melanoma; GSK’s Daprodustat will have to face FDA Advisory Committee; Breakthrough Therapy Status to Pfizer’s Group B Strep Vaccine; EU Approves Gilead’ Tecartus; Gilead’ Trodelvy Results in TROPiCs-02 Trial
Amgen Reveals the Top-line Result of its CodeBreak-200 trial of Lumakras in Lung Cancer The top-line result of Amgen's CodeBreak-200 trial of Lumakras in lung cancer was presented in abstract form at ESMO two weeks ago, showing a 34% improvement in progression-free survival (PFS) compared to chemotherapy. The fu...
Read More...
Aug 30, 2022
Aktis’s Novel Targeted Alpha Radiopharmaceuticals; Koye Partners with Sonde Health; Novartis to Spin Off Sandoz Business; Alcon to Buy Aerie Pharma; Fast Track Designation to Merck’s MK-2060; FDA Approves Ibrutinib for Chronic GvHD; French Authorities Clears BrainVectis’s Clinical Trial; Takeda’s Dengue Vaccine TAK-003 Gets Approval in Indonesia
Aktis Oncology Raises USD 84 Million To Advance Novel Targeted Alpha Radiopharmaceuticals Aktis Oncology has raised an additional USD 84 million in its Series A round, adding to the USD 72 million raised last year to help bring its radiopharmaceuticals to market. The extension to the first round included Merck's...
Read More...
Jul 05, 2022
AstraZeneca’s Imfinzi Shows Positive Results; Novartis Announces Results of Tislelizumab; FDA Grants Orphan Drug Designation to Evorpacept; EU Approves Sanofi’s Xenpozyme; Bayer’s Kerendia Approves in China; FDA Breakthrough Therapy Designation to Talquetamab; FDA Puts Clinical Hold on Sanofi’s Tolebrutinib; FDA Rejects Spero’s Tebipenem
FDA Grants Orphan Drug Designation to Evorpacept for AML ALX Oncology Holdings announced that the U.S. Food and Drug Administration had granted orphan drug designation to Evorpacept, a next-generation CD47 blocker, for treating patients with acute myeloid leukemia (AML). Acute Myeloid Leukemia (AML) is an agg...
Read More...
Jun 21, 2022
Biogen terminates ALS Pact with Karyopharm; AbbVie’s Immunological Drug Skyrizi; NICE Backs Astellas’ Oral Therapy Evrenzo; Roche’s Crenezumab Fails in Clinical Trial; FDA Grants Fast Track Designation to Dianhydrogalactitol; Sierra Oncology Submits NDA for Momelotinib
Biogen terminates USD 217 Million ALS Pact with Karyopharm Biogen has backed out of the four-year-old partnership with Karyopharm on a drug candidate for the neurological disease amyotrophic lateral sclerosis, which could have cost the US biotech up to USD 217 million. The 2018 agreement that granted Biogen righ...
Read More...
Feb 22, 2022
Sandoz’s Generic Revlimid; Agios’ Pyrukynd; Organon Announces 4Q & Full-year Earnings Report; BMS’ CAR-T Drug Breyanzi; Sanofi & Regeneron’s Dupixent Trial; AZ & Daiichi’s Drug Enhertu; Bayer’s Drug Kerendia; Lilly Releases Mirikizumab Data
Sandoz Launches generic Revlimid in 19 European Countries, Bringing a Flood of Competition to BMS' Megablockbuster Since Bristol Myers Squibb acquired Celgene and its megablockbuster Revlimid, the company has been bracing for the day when the multiple myeloma superstar would face generic competition. Sandoz, a s...
Read More...
Jan 05, 2022
Assessment of Key Products that Got FDA Approval in Second Half (H2) of 2021
Although COVID-19 continued to dominate headlines in 2021, pharmaceutical companies did not cease developing new treatments this year. The US Food and Drug Administration maintained a rapid rate of new drug approvals this year, all while managing the urgent examination of COVID-19 tests, treatments, and vaccines un...
Read More...
Dec 23, 2021
Sky Medical’s Geko™ device; Fist Assist Devices obtains Breakthrough Device designation; Edwards obtains FDA approval; BD launched instrument for testing; GE acquires BK Medical; Zynex’ CM-1500; Foldax’s TRIA biopolymer heart valve
Sky receives FDA clearance to market the geko™ device for venous insufficiency and ischemia On December 16, 2021, Sky Medical Technology Ltd, a medical device company, got approval from the US Food and Drug Administration (FDA) 510(k) to market the geko™ device for growing microcirculatory bl...
Read More...









-Agonist.png)

