Chronic Lymphocytic Leukemia
Jan 10, 2025
The Rise of Molecular Glues: Transforming Protein-Protein Interactions into Therapeutic Opportunities
Molecular glues are a groundbreaking class of small molecules that are gaining significant attention in the field of drug discovery. These compounds offer a novel approach to modulating protein function, especially for challenging targets that have been difficult to address using traditional drug discovery methods....
Read More...
Dec 12, 2023
Liso-cel in TRANSCEND CLL 004: Unprecedented and Sustained Responses with Manageable Safety Profile, Signaling a Promising Advance in Relapsed or Refractory CLL Treatment
Date of Abstract presentation9th December 2023IndicationsChronic lymphocytic leukemia (CLL) and Small lymphocytic lymphoma (SLL)Abstract Number330Abstract typeOral TRANSCEND CLL 004 is the first pivotal, multicenter study of a CD19-directed CAR T cell therapy for patients with relapsed or refractory chronic lymp...
Read More...
Dec 11, 2023
Eli’s Jaypirca: A Beacon of Success in the BTK Inhibitor Market
As the battle intensifies among AbbVie, Johnson & Johnson, AstraZeneca, and BeiGene in the BTK inhibitor market, Eli Lilly stands out by forging a new direction in the landscape of blood cancer drugs. Just at the beginning of this month, the FDA granted accelerated approval to Lilly’s Jaypirca. This approval is...
Read More...
Jan 24, 2023
BeiGene’s Brukinsa Approval; FDA Approval to Seagen’s TUKYSA; NICE Recommends Alnylam’s Amvuttra; FDA Approves Brenzavvy for Type 2 Diabetes; Roche’s Tecentriq to be Filed for Early-stage Liver Cancer; FDA Lifts Hold on Astellas’ Pompe Gene Therapy
FDA Approves BeiGene’s Brukinsa for CLL/SLL BeiGene's Brukinsa (zanubrutinib) for chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) has been approved by the US Food and Drug Administration. CLL is a common type of leukemia, accounting for approximately 25% of all new cases each year. SLL is...
Read More...
Jun 09, 2020
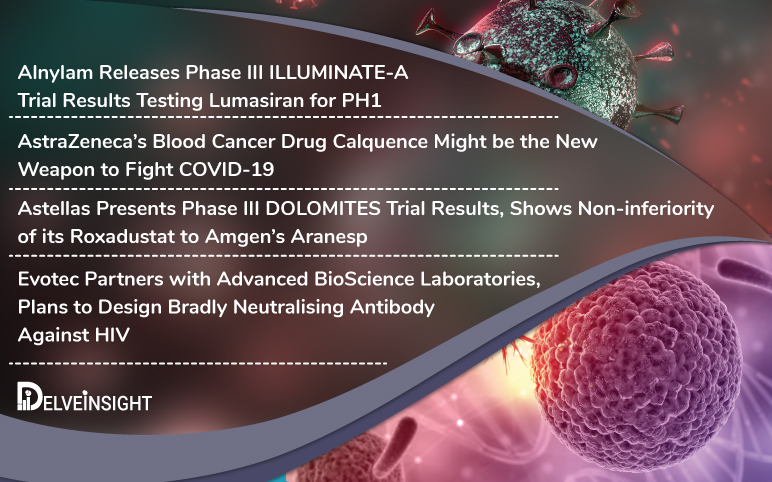
Alnylam’s lumasiran results; AZ’s oncology drug; Astellas Roxadustat; Evotec’s partnership with ABL
Alnylam Pharmaceuticals has released the Phase III results of the clinical trial ILLUMINATE-A evaluating lumasiran for Primary Hyperoxaluria Type 1 Primary Hyperoxaluria Type 1 (PH1) is a rare disorder that affects kidneys due to an excess buildup of oxalate, which in normal cases is filtered through the kidneys...
Read More...

-Agonist.png)

