Chronic Obstructive Pulmonary Disease
Dec 10, 2024
AbbVie Reveals Phase III TEMPO-2 Trial Positive Topline Results; FDA Accepts GSK’s NUCALA Submission in COPD; Novartis Boosts Huntington’s Disease Development Program with PTC518 In-Licensing; Chimerix to File for Accelerated FDA Approval of Dordaviprone for H3 K27M-Mutant Diffuse Glioma; MeiraGTx Receives FDA RMAT Designation for AAV2-hAQP1 in Grade 2/3 Radiation-Induced Xerostomia Treatment
AbbVie Unveils Positive Phase III TEMPO-2 Trial Findings for Tavapadon as Parkinson's Disease Monotherapy AbbVie announced favorable topline results from its pivotal Phase III TEMPO-2 trial, which assessed the investigational drug tavapadon as a flexible-dose monotherapy for early-stage Parkinson's disease. Tava...
Read More...
Oct 01, 2024
IntraBio’s AQNEURSA Niemann-Pick Disease Approval; FDA Approves Novel Schizophrenia Drug After 35 Years; Selpercatinib Gets FDA Nod for RET-Mutated MTC; DUPIXENT Receives First-Ever COPD Approval; Pfizer Withdraws OXBRYTA for Sickle Cell Disease from Global Market
IntraBio's AQNEURSA Receives Historic FDA Approval for Niemann-Pick Disease Type C Treatment IntraBio Inc. has received approval from the FDA for AQNEURSA (levacetylleucine), marking a significant milestone in the treatment of neurological manifestations of Niemann-Pick disease type C in both adults and pe...
Read More...
Sep 10, 2024
Biogen’s SPINRAZA Phase II/III Trial Results; Travere’s FILSPARI FDA Approval; GSK’s NUCALA Succeeds in COPD Trial; Summit’s NSCLC Win Over KEYTRUDA Raises Caution; FDA Lifts Hold on RZ358 for Congenital Hyperinsulinism
Biogen's Higher SPINRAZA Dose Shows Improved Efficacy in Phase II/III Trial A trial studying a higher dose of Biogen’s spinal muscular atrophy drug SPINRAZA (nusinersen) has met the primary endpoint in a cohort of infants with SMA. The Phase II/III DEVOTE study, which included 145 patients across various ages an...
Read More...
Jul 09, 2024
Lilly’s Morphic Acquisition; IDEAYA’s IDE397 Positive Phase II Trial Result; XPOVIO (selinexor) Approval in China; Roche to Reintroduce Susvimo in the US; Dupixent EU Approval
Lilly Strengthens IBD Treatment Portfolio with Morphic Acquisition Eli Lilly and Company and Morphic Holding, Inc. announced a definitive agreement for Lilly to acquire Morphic, a biopharmaceutical company developing oral integrin therapies for serious chronic diseases. Lilly will initiate a tender offer to acqu...
Read More...
Jul 02, 2024
Eisai Announces Solo Development of Farletuzumab Ecteribulin (FZEC); Johnson & Johnson’s Nipocalimab Phase III Trial; Merck’s WINREVAIR EU CHMP Recommendation; Verona Pharma’s Ohtuvayre FDA Approval; AstraZeneca’s Lynparza and Imfinzi EU Approval
Eisai Announces Solo Venture for Farletuzumab Ecteribulin (FZEC) Antibody Drug Conjugate Eisai Co., Ltd. announced the termination of its global strategic collaboration with Bristol Myers Squibb for the co-development and co-commercialization of farletuzumab ecteribulin (FZEC), previously known as MORAb-202, an ...
Read More...
Jan 24, 2024
Airway Management Devices: Charting the Evolving Market Trends and Key Innovations
Technological evolution has significantly transformed Airway Management Devices in recent years, ushering in a new era of efficiency, precision, and patient safety. Advances in materials, design, and manufacturing processes have led to the development of more sophisticated devices. Moreover, the incorporation of hi...
Read More...
May 31, 2023
ATS 2023 Updates: Dupixent – A Ray of Hope For Moderate To Severe Chronic Obstructive Pulmonary Disease (COPD)
DUPIXENT has shown positive pivotal results for COPD, confirming the key role of IL-4 and IL-13 in type 2 inflammatory diseases. DUPIXENT has the potential to be the first biologic with unprecedented and paradigm-shifting clinical results to treat COPD, having demonstrated a statistically significant reduc...
Read More...
Apr 11, 2023
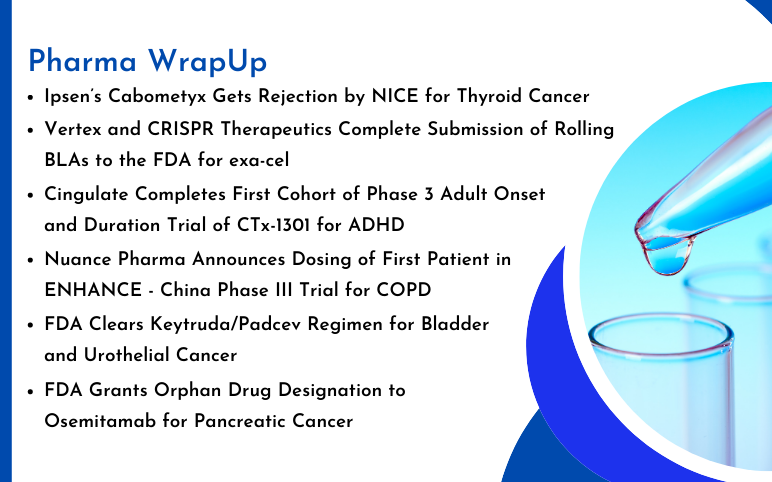
Ipsen’s Cabometyx Rejected by NICE; Vertex and CRISPR Therapeutics’s Submit BLA to the FDA for exa-cel; Orphan Drug Designation to Osemitamab for Pancreatic Cancer; FDA Clears Keytruda/Padcev for Bladder and Urothelial Cancer; Cingulate Completes Trial of CTx-1301 for ADHD; Nuance Pharma Announces Dosing of First Patient in ENHANCE Trial
FDA Grants Orphan Drug Designation to Osemitamab for Pancreatic Cancer Transcenta Holding Limited has announced that the U.S. Food and Drug Administration (FDA) has awarded Orphan Drug Designation to Osemitamab (TST001), a highly potent humanized monoclonal antibody that enhances ADCC (antibody-dependent cell-me...
Read More...
Nov 29, 2022
C4X Discovery and AstraZeneca Signs Deal; FDA Rejects Spectrum’s Poziotinib; Orphan Drug Designation to Tenaya’s Gene Therapy; EC Approves Regeneron’s Libtayo; Response Letter to Poziotinib for Metastatic NSCLC Harboring HER2 Exon 20 Mutations; Japan Approves Trastuzumab Deruxtecan for HER2+ Breast Cancer
C4X Discovery Holdings and AstraZeneca Signs Exclusive USD 402 Million Global License C4X Discovery Holdings has signed an exclusive global license with AstraZeneca worth up to USD 402 million for the development and commercialization of the NRF2 Activator program. The agreement will allow AstraZeneca to develop...
Read More...
Aug 30, 2022
Aktis’s Novel Targeted Alpha Radiopharmaceuticals; Koye Partners with Sonde Health; Novartis to Spin Off Sandoz Business; Alcon to Buy Aerie Pharma; Fast Track Designation to Merck’s MK-2060; FDA Approves Ibrutinib for Chronic GvHD; French Authorities Clears BrainVectis’s Clinical Trial; Takeda’s Dengue Vaccine TAK-003 Gets Approval in Indonesia
Aktis Oncology Raises USD 84 Million To Advance Novel Targeted Alpha Radiopharmaceuticals Aktis Oncology has raised an additional USD 84 million in its Series A round, adding to the USD 72 million raised last year to help bring its radiopharmaceuticals to market. The extension to the first round included Merck's...
Read More...






-Agonist.png)

