Cushing's syndrome
Mar 04, 2025
PRGN-2012 Wins Priority Review for Recurrent Respiratory Papillomatosis; PYX-201 Granted Fast Track for R/M HNSCC; AJA001 IND Cleared for Autism Spectrum Disorder; LAE120 IND Approved for Advanced Solid Tumors; Relacorilant NDA Accepted for Hypercortisolism Treatment
FDA Grants Priority Review to Precigen’s BLA for PRGN-2012 in Recurrent Respiratory Papillomatosis Precigen, Inc. announced that the FDA has accepted its Biologics License Application (BLA) for PRGN-2012 (zopapogene imadenovec†), an investigational AdenoVerse gene therapy for adults with recurrent respiratory pa...
Read More...
Nov 05, 2024
Prolong’s PP-007 Fast-Tracked for Stroke; FDA Expands JYLAMVO Pediatric Approval; Corcept’s Cushing’s Drug Shows Positive Phase III Results; ESSA Halts Phase II Study of Masofaniten for Prostate Cancer; SCEMBLIX Approved for Leukemia
Prolong Pharmaceuticals Secures FDA Fast Track for PP-007 in Stroke Therapy Prolong Pharmaceuticals, LLC, a clinical-stage biopharmaceutical company, announced that its investigational therapy, PP-007 (PEGylated carboxyhemoglobin, bovine), has been granted Fast Track designation by the FDA for the treatment of a...
Read More...
Feb 06, 2017
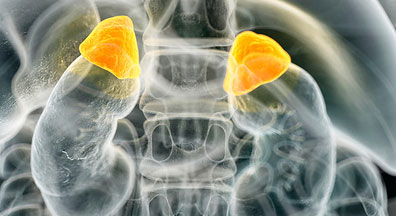
Cushing’s Syndrome: Cortisol Plethora
Cushing’s syndrome, also known as hypercortisolism, is an endocrine disorder that occurs due to abnormally high levels of the hormone cortisol production in the body. Cushing’s syndrome is broadly classified as endogenous and exogenous Cushing’s syndrome. Exogenous Cushing’s syndrome occurs due to excessive use of m...
Read More...


-Agonist.png)

