Daiichi Sankyo
Jul 25, 2023
Gilead’s Magrolimab Plus Azacitidine for MDS; FDA Approveds VANFLYTA for Newly Diagnosed AML; FDA Awards Fast Track Designation to ARX517 mCRPC; EMA Rejects Mirati’s Krazati; Harmony Phase III Pitolisant Trial for PWS Patients; Belite Bio’s Phase 3 DRAGON Trial of Tinlarebant for STGD
Gilead To Discontinue Phase III ENHANCE Study of Magrolimab Plus Azacitidine in Higher-Risk MDS Gilead Sciences, Inc. reported that the Phase III ENHANCE study in higher-risk myelodysplastic syndromes (MDS) has been halted due to futility based on a planned analysis. The safety data in this trial are consistent ...
Read More...
Jun 02, 2023
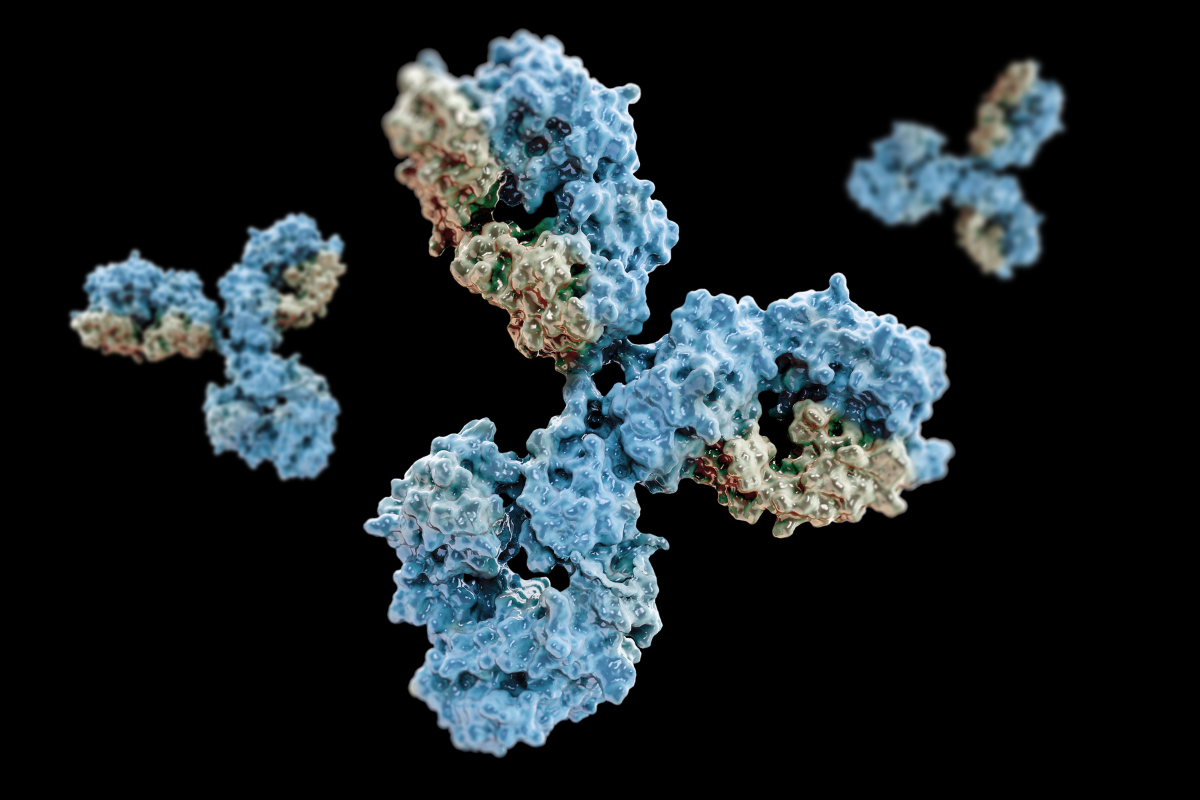
Antibody-drug Conjugates in Oncology: An Overview of the Current and Future Treatment Landscape
Antibody-drug conjugates (ADCs) are becoming more prominent in the cancer treatment market. Antibody-drug conjugates are one of the most rapidly increasing anticancer treatments. This approach uses a mAb attached to a cytotoxic payload via a chemical linker that is directed towards a target antigen expressed on the...
Read More...
Apr 25, 2023
Janssen’s AKEEGA Approval; FDA Approves Roche’s Polivy Combo for Frontline B-cell Lymphoma; Daiichi Sankyo’s Quizartinib for Adults With FLT3-ITD-Positive AML; bluebird bio BLA for lovo-cel for Patients with Sickle Cell Disease; Fast Track Designation for Lu-PNT2002 for mCRPC Treatment; FDA Orphan Drug Designation to XORTX’s Oxypurinol
Janssen Marks First Approval Worldwide for AKEEGA® (Niraparib and Abiraterone Acetate Dual Action Tablet) The Janssen Pharmaceutical Companies of Johnson & Johnson announced that the European Commission (EC) had granted marketing authorization for AKEEGA® (niraparib and abiraterone acetate [AA]), in the form...
Read More...
Jan 19, 2021
Biohaven’s Troriluzole Failure; Daiichi/ AZ’s Enhertu; Fujifilm & Manufacturing Spree; J&J’s Darzalex Faspro
Biohaven's Troriluzole Dwindles Again In Alzheimer’s After Anxiety Biohaven Pharmaceuticals had put too much faith in its third-generation prodrug, Troriluzole. The company has tested the efficacy of the drug in more than one indication, including generalized anxiety disorder (GAD), obsessive-compulsive di...
Read More...
Sep 10, 2019
Daiichi Sankyo fuels commercialization of DS-8201 in Japan; Roivant Sciences transfers its biotech stake for USD 3 Billion; FDA, EMA accepts marketing of Allergan’s abicipar
Daiichi Sankyo announced the submission of a New Drug Application (NDA) of DS-8201 to Japan's Ministry of Health, Labour and Welfare (MHLW). DS-8201 [fam-] trastuzumab deruxtecan), is an investigational antibody-drug conjugate (ADC) designed to deliver cytotoxic chemotherapy to cancer cells via a human epiderma...
Read More...
Sep 03, 2019
Helsinn out licenses Pracinostat in South America
Helsinn Group grants exclusive licensing rights to Blanver and Varifarma for its leading drug Pracinostat in South America. Helsinn is a Swiss biopharmaceutical company committed to producing the best solutions aimed at improving the quality of life for people with cancer. Under the terms of the agreements, ...
Read More...
Aug 06, 2019
FDA Approves Turalio; Sosei Heptares and Takeda Forge R&D Partnership
FDA approves first Tenosynovial giant cell tumour treatment Turalio (Pexidartinib) The US FDA has given its approval to Turalio (pexidartinib) capsules for the treatment of symptomatic tenosynovial giant cell tumour (TGCT) associated with severe morbidity or functional limitations that surgery has failed to impr...
Read More...
May 17, 2018
Business Cocktail
Zymeworks Daiichi I-O deal could potentially be worth USD 485 million Zymeworks and Daiichi Sankyo teamed up in 2016 on bispecific antibodies at that time the financial details were kept under the curtains. The pair is now entering into a new license agreement with a potential deal worth of around USD 484.7 million....
Read More...
Feb 24, 2017
Colony Stimulating Factor 1 Receptor (CSF1R) and Its connection with Memory Retention
Colony Stimulating Factor 1 Receptor (CSF1R) is a cell-surface protein encoded, in humans, by the CSF1R gene. CSF1R is also known as macrophage colony-stimulating factor receptor (M-CSFR), and CD115 (Cluster of Differentiation 115), and is encoded by CSF1R gene which controls the production, differentiation, and fun...
Read More...





-Agonist.png)

