Diabetes
Jan 17, 2025
Everything You Need to Know About Acute Kidney Injury
Acute Kidney Injury (AKI) is a significant medical condition that affects millions of individuals worldwide each year. It is a rapidly progressive condition that requires timely diagnosis and treatment to prevent severe complications or long-term consequences. AKI is particularly prevalent in hospitalized patients,...
Read More...
Sep 27, 2024
Promising Data from the First Dedicated Kidney Outcomes Trial with GLP-1 Receptor Agonist, Semaglutide, in Patients with Type 2 Diabetes and Chronic Kidney Disease
On 11 September 2024, the latest results from the FLOW trial were presented at the European Association for the Study of Diabetes (EASD) conference in Madrid, Spain. The FLOW trial, initiated in 2019, was a global, multinational, randomized controlled trial conducted across 28 countries, 387 sites, and 3,533 partic...
Read More...
Sep 27, 2024
Pooled Analysis of Finerenone Presented at EASD 2024, Poised to Strengthen its Position in the Cardio-Kidney-Metabolic Market Further
Data from an integrated pooled analysis of finerenone across three Phase III trials involving heart failure, chronic kidney disease (CKD), and Type 2 diabetes (T2D) was presented at the 2024 European Association for the Study of Diabetes (EASD) Congress. This analysis was conducted with data from the FIDELIO-DKD2 a...
Read More...
Sep 12, 2024
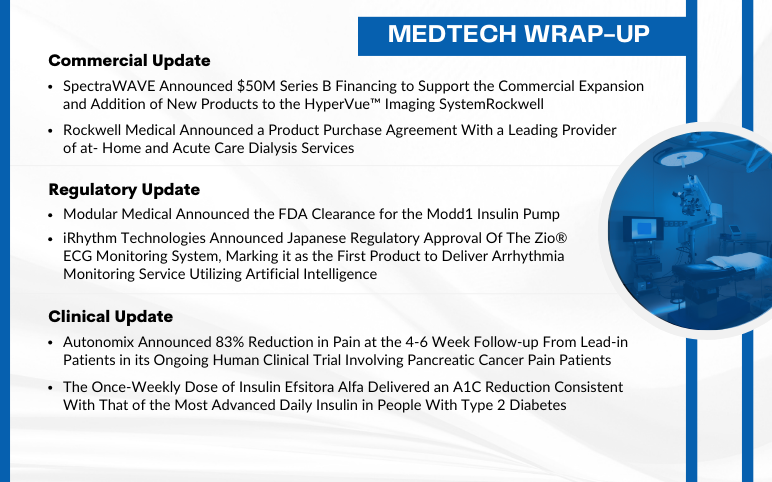
SpectraWAVE raised $50M; Rockwell Medical signed dialysis deal; Modular Medical got FDA nod for Modd1 pump; iRhythm’s Zio approved in Japan; Autonomix trial showed 83% pain reduction; Insulin Efsitora Alfa improved A1C.
Modular Medical Announced the FDA Clearance for the Modd1 Insulin Pump On September 5, 2024, Modular Medical, Inc., an insulin delivery system technology company, announced that it received the U.S. Food and Drug Administration (FDA) clearance to market and sell its MODD1 pump in the United States. With it...
Read More...
Jul 23, 2024
CEPI Grants $41.3 Million to Valneva; Innovent Achieves Phase III Success for Mazdutide; GSK’s BLENREP Combination Therapies EMA Review Application; Darolutamide Phase III ARANOTE Trial; Roche’s SUSVIMO Shows Long-Term Efficacy
CEPI Grants $41.3 Million to Valneva to Enhance Global Access to First Chikungunya Vaccine The Coalition for Epidemic Preparedness Innovations and Valneva SE have expanded their partnership to enhance access to the world's first chikungunya vaccine, IXCHIQ®, in Low- and Middle-Income Countries (LMICs). CEPI will...
Read More...
Jul 16, 2024
Immutep’ First-Line Treatment Positive Outcomes; Pfizer’s Once-Daily Oral GLP-1 Agonist Danuglipron; FDA Issues Complete Response Letter to Novo Nordisk; Arcutis’ ZORYVE® Cream 0.15% FDA Approval; NICE Recommends Ebglyss For Moderate To Severe Atopic Dermatitis
Immutep Announces Promising Outcomes for First-Line Treatment in PD-L1 Negative Head and Neck Squamous Cell Carcinoma Patients Immutep Limited announced positive results from Cohort B of the TACTI-003 (KEYNOTE-PNC-34) Phase IIb trial, evaluating eftilagimod alfa (efti) combined with MSD’s anti-PD-1 therapy KEYTR...
Read More...
Jun 25, 2024
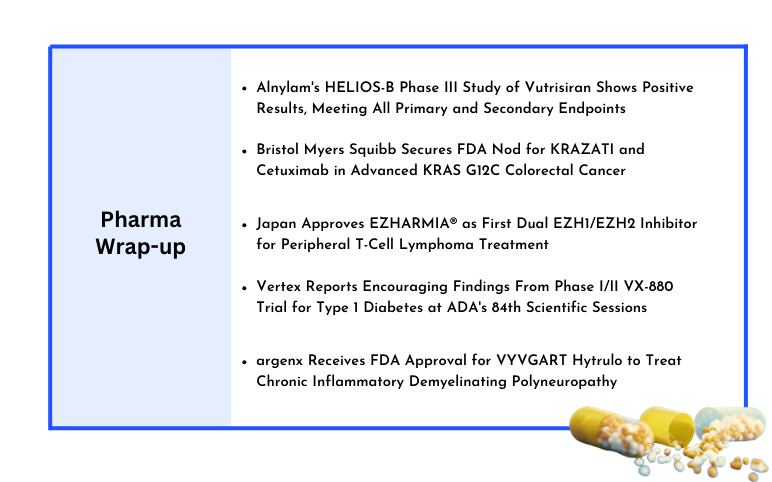
Alnylam’s HELIOS-B Phase III Study of Vutrisiran; Bristol Myers Squibb Secures FDA Nod for KRAZATI and Cetuximab; Daichii Sankyo’s EZHARMIA® Receives Japan Approval; Vertex’s Phase I/II VX-880 Trial; argenx’s VYVGART Hytrulo FDA Approval
Alnylam's HELIOS-B Phase III Study of Vutrisiran Shows Positive Results, Meeting All Primary and Secondary Endpoints Alnylam Pharmaceuticals, Inc. reported encouraging topline outcomes from its HELIOS-B Phase III study of vutrisiran, an experimental RNAi therapy being developed to treat ATTR amyloidosis with car...
Read More...
Apr 03, 2024
Nutrition Nexus: Illuminating the Growth Trajectory Path in the Dietary Supplements Market
The Dietary Supplements market has experienced significant growth over the past years, driven by a variety of factors contributing to its sustained demand. One primary reason for this surge is the increasing awareness and emphasis on preventive healthcare and wellness among consumers worldwide. As people become mor...
Read More...
Dec 25, 2023
Future Avenues for Prediabetes Treatment: The Road Ahead
Prediabetes is a significant health concern characterized by elevated blood sugar levels that fall between the normal range and the diagnostic threshold for type 2 diabetes. Often labeled as borderline diabetes, this metabolic condition is intricately linked to the global rise in obesity and poses an escalating wor...
Read More...
Dec 05, 2023
FDA Grants Priority Review to Merck’s Application for KEYTRUDA Plus Padcev; Roche and Carmot Therapeutics’s Definitive Merger Agreement; AbbVie to Acquire ImmunoGen; FDA Grants Orphan Drug Designation to LP-284; Merck Announces Commercialization Agreement With Abbisko; Pfizer and Valneva Complete Recruitment for Phase 3 VALOR Trial
FDA Grants Priority Review to Merck’s Application for KEYTRUDA Plus Padcev for the First-Line Treatment of Patients With Locally Advanced or Metastatic Urothelial Cancer Merck, operating as MSD internationally, reported that the U.S. Food and Drug Administration (FDA) has prioritized the review of a supplementar...
Read More...






-Agonist.png)

