Diabetes
Oct 25, 2023
Charting the Growth of the Insulin Pens Market Amidst Rising Demand
The evolution of technology has been a driving force behind the remarkable growth of the Insulin Pens market. Insulin Pens, from their inception as relatively basic delivery devices, have undergone a substantial transformation, becoming highly advanced and user-friendly tools. In the early stages, these pens requir...
Read More...
Sep 28, 2023
Orthofix Launched the Galaxy Fixation Gemini System; Oxford Biodynamics Launched the EpiSwitch Prostate Screening Blood Test; CE Mark for the Medtronic’s New Simplera CGM; FDA 510(k) Clearance to MicroVention’s SOFIA™ EX 5F 115cm; SHL Telemedicine’s SmartHeart® Technology; Cardiosense’s Nationwide Heart Failure Study
Orthofix Launched the Galaxy Fixation Gemini System and Expanded Sterile Kit Offerings for Orthopedic Trauma Procedures On September 20, 2023, Orthofix Medical Inc., a leading global spine and orthopedics company, launched the Galaxy Fixation Gemini™ system. It is a stable external fixation system that ...
Read More...
Sep 27, 2023
The Revolution of Healthcare: Smart Medical Devices Transforming the Future
It is not surprising that the healthcare sector is undergoing a significant transition in an era characterized by technological developments and the digitization of almost every aspect of our lives. The development of smart medical devices is a shining illustration of how technology has advanced in healthcare in a ...
Read More...
May 01, 2023
Cell and Gene Therapies for Diabetes Treatment: A Permanent Cure for Patients?
Diabetes is the 8th largest cause of death in the United States (although its prevalence may be underreported). Diabetes affects more than 37 million people in the United States, and 1 in every 5 are unaware of their condition. Over 96 million US adults—more than one-third—have prediabetes, and more than 8 out of 1...
Read More...
Jan 26, 2023
Tandem Acquires AMF Medical; NeuroMetrix Launched DPNCheck 2.0 Device; FDA Approves Cytovale’s IntelliSep Sepsis Test; FDA Clearance for the Ø 4.5mm VADER Pedicle Screws; Pie Medical Imaging’s vFFR FASTIII Trial; Hip Innovation Technology Announced Initiation Investigational Device Exemption Study
Tandem Diabetes Care Completed Acquisition of the Insulin Patch Pump Developer, AMF Medical On January 23, 2023, Tandem Diabetes Care, Inc., a global insulin delivery and diabetes technology company, announced the complete acquisition of AMF Medical SA, the privately held Swiss developer of the SigiTM Patch Pump...
Read More...
Dec 01, 2022
AnchorDx’s UriFind Bladder Cancer Assay in the US; UroMems Initiates Smart Implant to Treat Stress Urinary Incontinence; FDA 510(k) Clearance to NeuroLogica’s BodyTom 64; CE-IVD Mark Approval to SeekInCure’s Recurrence Monitoring Kit Gets; Ypsomed and CamDiab’s Automated Insulin Dosing System; Boston Scientific to Acquire Apollo Endosurgery
AnchorDx Clinical Trial Enrols First Patient for its UriFind® Bladder Cancer Assay in the US On November 23, 2022, AnchorDx, announced the first patient enrollment for its clinical trials of the UriFind® bladder cancer assay in the United States. The UriFind® bladder cancer assay clinical trial in the U...
Read More...
Nov 22, 2022
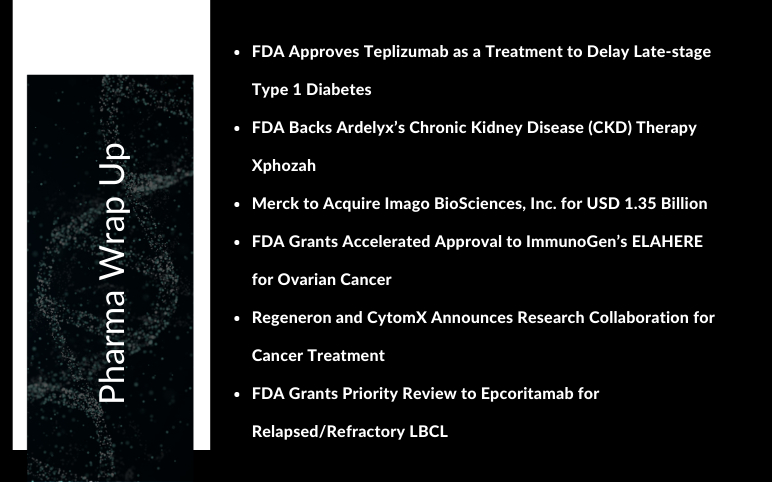
FDA Approves Teplizumab to Delay the Onset of Type 1 Diabetes; FDA Backs Ardelyx’s CKD Therapy Xphozah; Merck to Acquire Imago BioSciences; Accelerated Approval to ImmunoGen’s ELAHERE; Regeneron and CytomX Announces Research Collaboration; FDA Grants Priority Review to Epcoritamab R/R LBCL
Merck to Acquire Imago BioSciences, Inc. for USD 1.35 Billion While its rumored acquisition of Seagen has yet to materialize, Merck & Co has continued to bolster its pipeline with smaller transactions, the most recent of which was a USD 1.35 billion agreement to acquire Imago BioSciences and its bomedemstat ...
Read More...
Nov 17, 2022
Medtronic Launches Infusion Set for Insulin Pumps; Penumbra’s Virtual Reality-Based Rehabilitation System; FDA Approval to Genesis’s Chocolate Touch® Drug-coated Balloon PTA Catheter; FDA Nod to AEYE’s AI-based Autonomous Screening; Alpheus Medical Treats First High-Grade Glioma Brain Cancer Patient with its Proprietary Platform; Karidum’s Next Generation Globe® Pulsed Field System
Medtronic Launches World's First and Only Infusion Set for Insulin Pumps that Doubles Wear Time up to 7 days in the US On November 15, 2022, Medtronic plc, a global leader in healthcare technology, announced the US launch of the Medtronic Extended infusion set, the first and only infusion set labeled for up to ...
Read More...
Nov 16, 2022
Insights Into the Key Advancements Transforming the Type 2 Diabetes Treatment and Management
Diabetes is a chronic metabolic disorder that is influenced by a wide variety of factors and is characterized by high or low blood glucose (or blood sugar) levels in the body. Diabetes is a major risk factor for blindness, kidney failure, heart attacks, stroke, and lower limb amputation. Also, it can significantly ...
Read More...
Aug 18, 2022
CereVasc’s eShunt System Study; FDA Approves NGS-Based CDx for Trastuzumab Deruxtecan; Nanopath Secures $10 Million Funding; BD, Accelerate Diagnostics Announce Collaboration; Avails Medical’s Clinical Trials for eQUANT; Movano Ring Exceeds Accuracy Targets for SpO2 & Heart Rate Monitoring
CereVasc Announces FDA Approval of Second IDE Study of the eShunt® System On August 09, 2022, CereVasc, Inc., a privately held, clinical-stage medical device company developing novel, minimally invasive treatments for neurological diseases, announced that the U.S. Food and Drug Administration (FDA) has approved ...
Read More...





-Agonist.png)

