Eosinophilic Esophagitis
Oct 07, 2024
DUPIXENT Receives First-Ever Biologic Approval for COPD: Adds Another Jewel in its Crown
After more than ten years without significant therapeutic advancements in chronic obstructive pulmonary disease (COPD), patients with this progressive condition now have two new treatment options, with more likely to follow. Regeneron and Sanofi recently announced that the FDA has broadened the use of the highly...
Read More...
Feb 09, 2024
Dupixent Breaks Ground: First and Only Eosinophilic Esophagitis Treatment for Pediatric Patients
Sanofi and Regeneron are prioritizing pediatric care, particularly in their recent progress with the potent anti-inflammatory drug, Dupixent. On January 25, 2024, the FDA approved Dupixent, an IL-4 receptor alpha antagonist, for the treatment of eosinophilic esophagitis (EoE) in children aged 1 to 11, weighing at l...
Read More...
Jan 30, 2024
Merck’s KEYTRUDA as Adjuvant Therapy for RCC Patients; BMS Receives Positive CHMP Opinion for CAR T Cell Therapy Abecma for Multiple Myeloma; FDA Approves Dupixent for Eosinophilic Esophagitis; Juvena Receives FDA Orphan Drug Designation for JUV-161; European Commission Authorizes GSK’s Omjjara; ENHERTU Granted Priority Review in the US for for metastatic HER2-positive Solid Tumors
Merck’s KEYTRUDA Reduced the Risk of Death by 38% Versus Placebo as Adjuvant Therapy for Patients With Renal Cell Cancer (RCC) at an Increased Risk of Recurrence Following Nephrectomy Merck, also known as MSD beyond the United States and Canada, has revealed findings from the Phase III KEYNOTE-564 trial, which a...
Read More...
Jan 09, 2023
Is it the Dawn of Biologics in the Eosinophilic Esophagitis Treatment Landscape?
Eosinophilic Esophagitis (EoE) is a chronic inflammatory ailment that is immune-mediated. It is characterized by an increased number of eosinophils infiltrating the esophagus, thus, impacting one’s fundamental eating ability. It affects both adults and children and is caused by allergens; food antigens are consider...
Read More...
May 24, 2022
PTC Therapeutics’ Gene Therapy Upstaza; Sanofi and Regeneron’s Dupixent; Bayer CAR-T Collaboration with Atara; FDA Accepts Biohaven’s Zavegepant; AbbVie Files FDA Approval for ABBV-951; Innoviva to Acquire Entasis; FDA Orphan Drug Designation to XMT-2056; FDA Approves Azacitidine for Juvenile Myelomonocytic Leukemia
EU Recommends Approval for PTC Therapeutics’ Gene Therapy Upstaza Upstaza, a gene therapy developed by PTC Therapeutics for patients with the genetic condition AADC deficiency, has been recommended for EU approval, putting another test of gene therapy's commercial prospects in the union. Upstaza (eladocagene exu...
Read More...
Apr 05, 2022
Precigen’s PRGN-3006; Yescarta Approved as a First CAR T-cell Therapy for R/R LBCL; Biogen & Ionis’ BIIB078; Nobelpharma’s HYFTOR (sirolimus topical gel) 0.2%; Cerevance Parkinson’s Drug; Sanofi & Regeneron’s Dupixent; Clovis’s Rubraca for Ovarian Cancer; Immunocore Eye Cancer Cell Therapy Approval
FDA Approves Yescarta as a First CAR T-cell Therapy for Initial Treatment of R/R Large B-cell Lymphoma Until now, existing CAR-T therapies have been reserved for patients with blood cancer who have tried multiple treatments. The FDA has approved Yescarta, a CD19-directed CAR-T therapy developed by Gilead Science...
Read More...
May 18, 2021
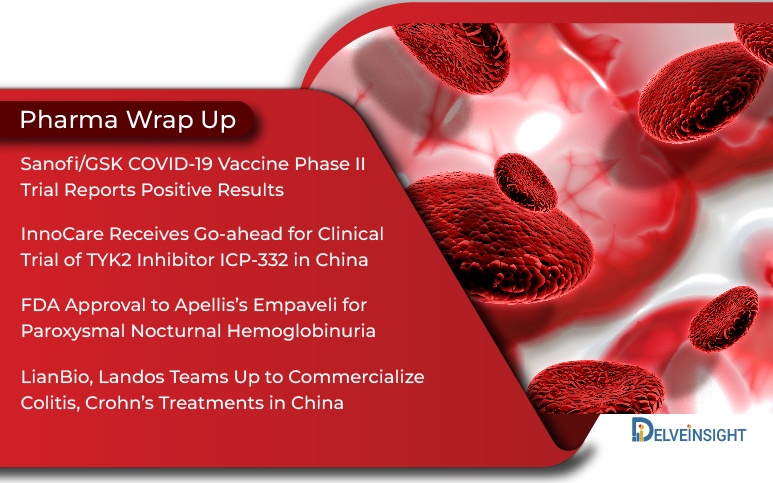
InnoCare’s Trial In China; Sanofi/GSK COVID-19 Vaccine; Apellis’s Empaveli for PNH; LianBio, Landos Collab
InnoCare Receives Go-ahead for Clinical Trial of TYK2 Inhibitor ICP-332 in China InnoCare, a biopharma firm developing novel therapies for the treatment of cancer and autoimmune diseases, today announced the approval of Phase I clinical trial of its novel TYK2 (Tyrosine Kinase 2) inhibitor, ICP-332, by the China...
Read More...




-Agonist.png)

