FDA
Mar 28, 2017
Tesaro puts AstraZeneca; XELJANZ® receives; Innovus nabs; Alexion brings
Tesaro puts AstraZeneca on notice with early FDA nod for Lynparza rival niraparib Tesaro’s closely watched ovarian cancer drug niraparib—now dubbed Zejula—won an FDA nod on 27th March, months before its scheduled decision date. Apart from getting approval, Zejula also got a broader label than its head-to-head rival,...
Read More...
Feb 28, 2017
Prosecutors rope Pfizer; Pharma groups to FDA; Regeneron simulates; Otsuka & Lundbeck revive;Philidor execs plead
Prosecutors rope Pfizer into fast-growing copay assistance probe After first focusing on biotech and speciality pharma, the feds have made their way to Big Pharma, with the New York drug giant joining a group that includes Gilead Sciences, Biogen, Valeant Pharmaceuticals and others about ties to copay assistance pro...
Read More...
Jan 27, 2017
Payers block EpiPen; Allergan charged; AbbVie’s Humira; Baxter paying $18M
Payers block Kaléo's expensive EpiPen challenger Kaléo reintroduced its Auvi-Q last week at a list price of $4,500 for a two-pack in an effort to capture some market share from Mylan’s EpiPen, which is listed at about $600 for a two-pack. Auvi-Q is set to launch next month. Under Kaléo’s pricing strategy, Auvi-Q wil...
Read More...
Dec 20, 2016
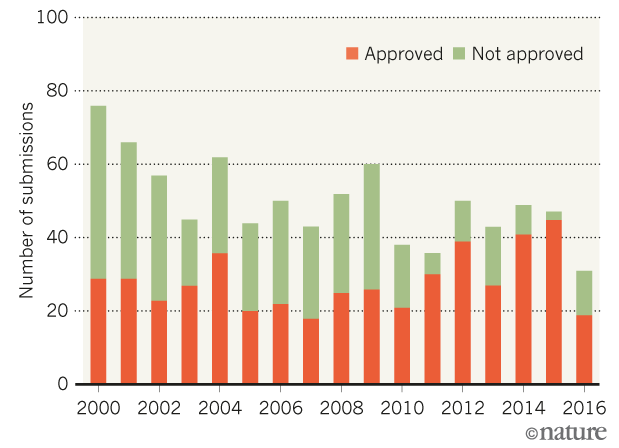
US drug approvals plummet in 2016
US drug approvals are on track to drop by more than half in 2016 compared to 2015. The agency had approved 19 new drugs this year as of 9 December, putting it on track for its lowest yearly tally since 2007. The decline is made more dramatic by 2015’s bumper crop of approvals. The FDA approved 45 new drugs last year...
Read More...
Nov 29, 2016
EC grants; Pfizer cuts; 27 medicines sold; Keytruda nabs
EC grants marketing authorisation for Takeda’s Ninlaro capsules The European Commission (EC) has granted conditional marketing authorisation for Takeda Pharmaceutical’s Ninlaro capsules. The oral proteasome inhibitor is indicated in combination with lenalidomide and dexamethasone for adult patients with multiple mye...
Read More...
Oct 04, 2016
FDA Approves STELARA; Novartis announces AMG 334; AbbVie’s HCV Regimen; PaizaBio Gains CFDA Approval; WuXi Biologics completes construction
FDA Approves Janssen's STELARA for the Treatment of Adults With Moderately to Severely Active Crohn’s Disease Janssen Biotech Inc. received approval from the U.S. FDA for STELARA (ustekinumab), used for the treatment of moderately to severely active Crohn’s disease in adults (18 years or older). The drug is for the ...
Read More...
Sep 30, 2016
The Snippet: The Success of Cannabidiol
GW Pharmaceuticals, a UK based company, believes in growing its own cannabis plants, search for its ingredients and then come up with medicines that help the company in taking one more step towards approval of Epidiolex, as seen in the announcement of positive phase 3 trial results earlier this week. The drug is fo...
Read More...
Sep 27, 2016
FDA grants approval; Sanaria receives fast-track designation; Sanofi and Regeneron’s next blockbuster; Allele receives NIH grant
US FDA grants approval for Novartis' Ilaris to treat Periodic Fever Syndromes Novartis received approval to market its Periodic Fever Syndrome drug Ilaris (cancakinumab). The drug targets the syndrome, and will be used to treat a group of diseases that cause serious recurrent fever and pathogenic inflammation throug...
Read More...
Sep 23, 2016
FDA Approves Sarepta’s Muscular Dystrophy Drug after Months of Debate
The Food and Drug Administration approved the recent controversial drug to treat Duchenne muscular Dystrophy, which is a rare disease that confines boys to wheelchairs and condemns them to an early death. The decision was made after months of debate between the Agency and Sarepta Therapeutics, regarding the evidence...
Read More...
Sep 07, 2016
Treatment for esophageal cancer; FDA bans soaps; Dr Reddy’s Launches; Cipla Gets Approval
Targeted treatment can become a reality for esophageal cancer, as a study opens door A group of researchers Scientists, funded by Cancer Research UK and Medical Research Council, have discovered that oesophageal cancer can be classified into three different subtypes, paving the way for testing targeted treatments ta...
Read More...





-Agonist.png)

