FDA
Oct 22, 2024
Gilead and Merck Announce Encouraging Phase II Results of Islatravir and Lenacapavir Combo; REGENXBIO Highlights Positive Data from Phase II ABBV-RGX-314 Wet AMD Study at AAO 2024; FDA Greenlights Astellas’ VYLOY for Advanced Gastric and GEJ Cancer Therapy; Merck’s CAPVAXIVE Vaccine Yields Strong Immune Response in Adults Vulnerable to Pneumococcal Disease; FDA Postpones LUMAKRAS Colorectal Cancer Verdict to Early 2025
Gilead and Merck Reports Phase II Data Showing that Switching to a Once-Weekly Oral Combination of Islatravir and Lenacapavir Maintained Viral Suppression in Adults Through 48 Weeks Gilead Sciences, Inc. and Merck have shared new findings from a Phase II clinical trial assessing the experimental combination of i...
Read More...
Oct 18, 2024
From NASH to MASH: Unraveling the Evolution and Future of Liver Disease Treatment
In the ever-evolving world of liver disease, the shift from Nonalcoholic Steatohepatitis (NASH) to Metabolic Associated Steatotic Hepatitis (MASH) marks a revolutionary leap in our understanding and approach to treatment. This transition isn't merely a rebranding; it signifies a profound recognition of the intricat...
Read More...
Oct 16, 2024
Transforming Pompe Disease Care: Latest Innovations and Strategic Advances in Treatment
Pompe disease is a rare genetic disorder that presents a spectrum of severity, with varying rates of progression and ages of onset. Symptoms can appear anywhere from infancy to late adulthood, with earlier onset generally associated with more rapid progression and increased severity. At all ages, the disease is mar...
Read More...
Oct 15, 2024
Astellas & AviadoBio’s Exclusive Deal for AVB-101; GSK’s Depemokimab Shows Positive Results for Nasal Polyps; Lilly’s Mirikizumab Outperforms Ustekinumab in Crohn’s Study; TREMFYA Delivers Strong Results in Crohn’s & Colitis; ENHERTU Approved in China for HER2-Mutant NSCLC.
Astellas & AviadoBio Sign Exclusive Deal for Gene Therapy AVB-101 in Frontotemporal Dementia AviadoBio Ltd. and Astellas Pharma Inc. have announced a strategic partnership under an exclusive option and license agreement for AVB-101, an investigational AAV-based gene therapy currently in Phase I/II developmen...
Read More...
Oct 01, 2024
IntraBio’s AQNEURSA Niemann-Pick Disease Approval; FDA Approves Novel Schizophrenia Drug After 35 Years; Selpercatinib Gets FDA Nod for RET-Mutated MTC; DUPIXENT Receives First-Ever COPD Approval; Pfizer Withdraws OXBRYTA for Sickle Cell Disease from Global Market
IntraBio's AQNEURSA Receives Historic FDA Approval for Niemann-Pick Disease Type C Treatment IntraBio Inc. has received approval from the FDA for AQNEURSA (levacetylleucine), marking a significant milestone in the treatment of neurological manifestations of Niemann-Pick disease type C in both adults and pe...
Read More...
Sep 24, 2024
Zevra’s MIPLYFFA Niemann-Pick Disease Approval; FASENRA Approved for Eosinophilic Granulomatosis; Sanofi’s SARCLISA Gets Multiple Myeloma Approval; RYBREVANT Combo Gets FDA Nod for EGFR Lung Cancer; UCB’s BIMZELX Receives Multiple Approval from the FDA
FDA Greenlights Zevra Therapeutics’ MIPLYFFA for Niemann-Pick Disease Type C Zevra Therapeutics, Inc. has announced that the FDA has approved MIPLYFFA (arimoclomol) capsules as the first oral treatment for Niemann-Pick disease type C. Indicated for use alongside miglustat, MIPLYFFA is intended for both adult and...
Read More...
Sep 19, 2024
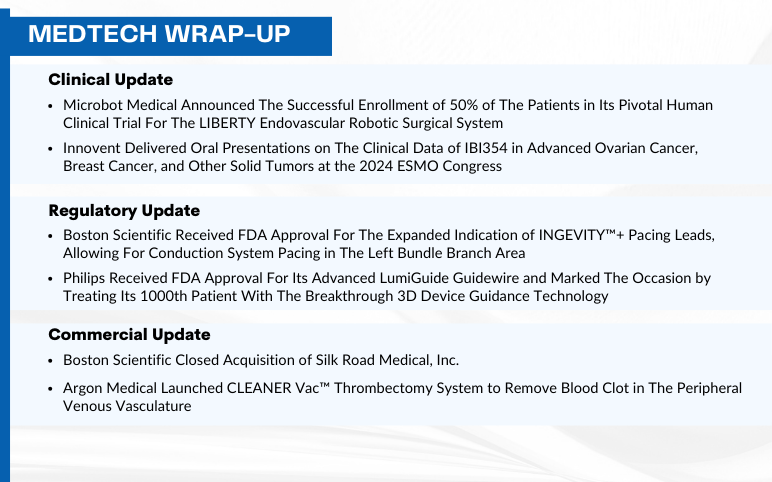
Boston Scientific INGEVITY™ gets Expanded Indication and acquires Silk Road Medical; FDA Approves Philips’ LumiGuide Guidewire; Microbot Completes LIBERTY Trial Enrollment; Innovent Presents Data at ESMO 2024; Argon Medical Launches CLEANER Vac™ System
Boston Scientific Received FDA Approval For The Expanded Indication of INGEVITY™+ Pacing Leads, Allowing For Conduction System Pacing in The Left Bundle Branch Area On September 17, 2024, Boston Scientific Corporation received U.S. Food and Drug Administration (FDA) approval to expand the indication for th...
Read More...
Sep 12, 2024
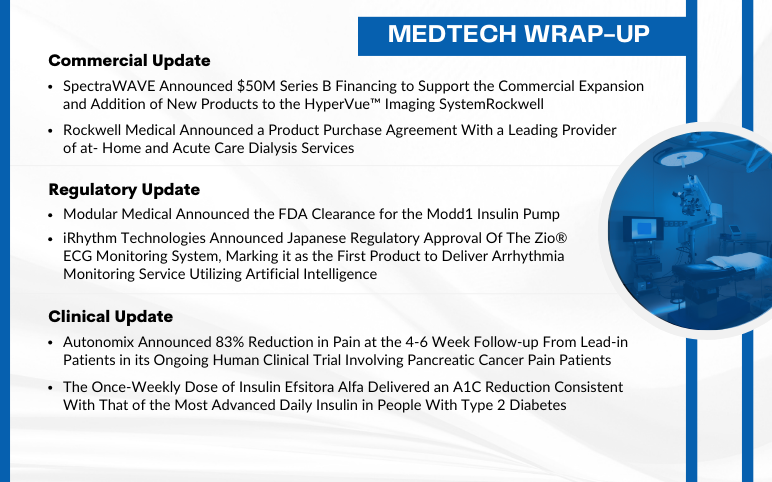
SpectraWAVE raised $50M; Rockwell Medical signed dialysis deal; Modular Medical got FDA nod for Modd1 pump; iRhythm’s Zio approved in Japan; Autonomix trial showed 83% pain reduction; Insulin Efsitora Alfa improved A1C.
Modular Medical Announced the FDA Clearance for the Modd1 Insulin Pump On September 5, 2024, Modular Medical, Inc., an insulin delivery system technology company, announced that it received the U.S. Food and Drug Administration (FDA) clearance to market and sell its MODD1 pump in the United States. With it...
Read More...
Sep 10, 2024
Biogen’s SPINRAZA Phase II/III Trial Results; Travere’s FILSPARI FDA Approval; GSK’s NUCALA Succeeds in COPD Trial; Summit’s NSCLC Win Over KEYTRUDA Raises Caution; FDA Lifts Hold on RZ358 for Congenital Hyperinsulinism
Biogen's Higher SPINRAZA Dose Shows Improved Efficacy in Phase II/III Trial A trial studying a higher dose of Biogen’s spinal muscular atrophy drug SPINRAZA (nusinersen) has met the primary endpoint in a cohort of infants with SMA. The Phase II/III DEVOTE study, which included 145 patients across various ages an...
Read More...
Sep 03, 2024
Bayer Phase III NSCLC Trial; D&D Pharmatech Gets FDA Nod for GLP-1R Agonist in Multiple Scletosis; Merck Halts Two KEYTRUDA Trials; Novartis Expands LEQVIO After Phase III Success; Alnylam Reports Strong Phase III Data for Vutrisiran
Bayer Starts Phase III Trial In Non-Small Cell Lung Cancer (NSCLC) Bayer has officially enrolled the first patient in the global Phase III SOHO-02 trial, which will evaluate the efficacy and safety of BAY 2927088 as a first-line treatment for advanced non-small cell lung cancer (NSCLC) with activating HER2 mutat...
Read More...








-Agonist.png)

