FDA
Apr 18, 2024
Baxter Introduced New Injectable Pharmaceuticals to US Market; Bayer and Hologic Collaborated to Deliver Contrast-Enhanced Mammography; Labcorp Received FDA Emergency Use Authorization for Mpox PCR Test Home Collection Kit; Onkos Surgical Won FDA De Novo Nod For Antibacterial-Coated Orthopedic Implants; Heartbeam Presented Positive Results On Its Artificial Intelligence Capabilities For Detecting Arrhythmias; Cognito Therapeutics Showed Positive Non-Invasive Neuromod Results
Baxter Introduced New Injectable Pharmaceuticals to US Market On April 11, 2024, the pharmaceutical portfolio of multinational medical goods corporation Baxter International Inc. was augmented with the introduction of five new injectable medications in the US market. The goods were made to fulfill essential dema...
Read More...
Apr 16, 2024
Roche’s Columvi Phase III STARGLO Trial; Novartis’ Fabhalta Latest Data; Vertex’s Alpine Immune Sciences Acquisition; Telix Pharmaceuticals’ TLX101-CDx Fast Track Designation; NovelMed’s NM5072 Orphan Drug Designation
Roche's Columvi Achieves Primary Endpoint of Prolonged Overall Survival in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma in Phase III STARGLO Trial Roche reported that the Phase III STARGLO trial successfully achieved its main goal of improving overall survival. The research revealed that in...
Read More...
Apr 11, 2024
Carl Zeiss Meditec AG’s Acquisition of Dutch Ophthalmic Research Center; Johnson & Johnson’s Shockwave Medical Acquisition; Biora Therapeutics’ BT-600 Positive Results; Medtronic’s Single-Shot Mapping Ablation Catheter Positive Data; Abbott’s TriClip FDA Approval; Abbott’s Whole Blood Rapid Test FDA Clearance
Carl Zeiss Meditec AG Completed Acquisition of Dutch Ophthalmic Research Center (D.O.R.C); Companies Unite to Shape Ophthalmology Market On April 04, 2024, Carl Zeiss Meditec AG announced that it acquired D.O.R.C. (Dutch Ophthalmic Research Center) from the investment firm Eurazeo SE, Paris, France. T...
Read More...
Apr 09, 2024
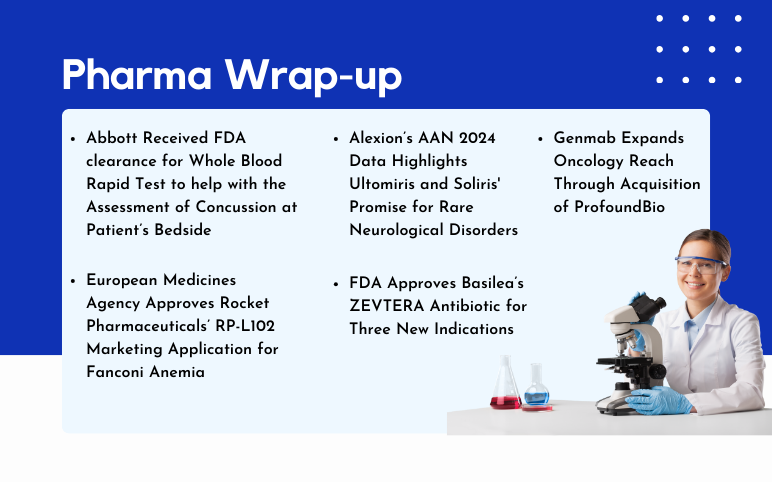
AstraZeneca and Daiichi Sankyo’s Enhertu US Approval; Basilea’s ZEVTERA FDA Approval; Genmab’s ProfoundBio Acquisition; Rocket Pharmaceuticals’ RP-L102 EMA Approval; Alexion’s Ultomiris and Soliris AAN 2024 Data
Enhertu Receives US Approval as First HER2-focused Treatment for Metastatic Solid Tumors, Independent of Tumor Origin AstraZeneca and Daiichi Sankyo's drug Enhertu (trastuzumab deruxtecan) has gained approval in the United States for treating adult patients with inoperable or metastatic HER2-positive (IHC 3+) so...
Read More...
Apr 04, 2024
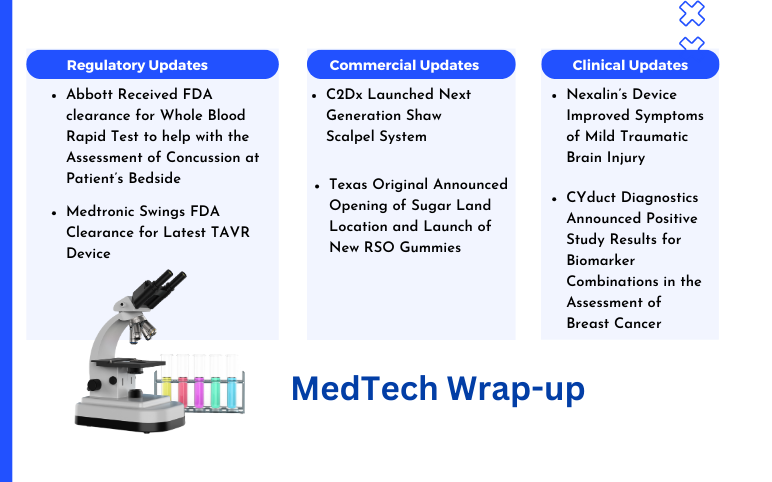
C2Dx’s Shaw Scalpel System; Texas Original’s RSO Gummies; Abbott’s Whole Blood Rapid Test FDA Clearance; Medtronic’s TAVR Device FDA Clearance; Nexalin’s Device Improved Symptoms; CYduct Diagnostics’ Positive Study Results
C2Dx Launched Next Generation Shaw Scalpel System On March 26, 2024, C2Dx, a privately-owned medical device company, announced the launch of its Next Generation Shaw Scalpel System. The Shaw Scalpel System has obtained 510(k) clearance from the FDA for the upgraded controller of its advanced surgical techn...
Read More...
Apr 02, 2024
AstraZeneca’s Voydeya FDA Approval; Akebia’s Vafseo FDA Approval; Bristol Myers Squibb’s Phase III YELLOWSTONE Trial Update; Astellas’ IZERVAY FDA Approval; AstraZeneca’s Truqap and Faslodex MHLW Approval
Voydeya Receives FDA Approval as Supplemental Treatment with Ravulizumab or Eculizumab for Managing Extravascular Hemolysis in Adult Patients with PNH Voydeya (danicopan) has received approval in the United States for use alongside ravulizumab or eculizumab in treating extravascular hemolysis (EVH) in adults dia...
Read More...
Mar 28, 2024
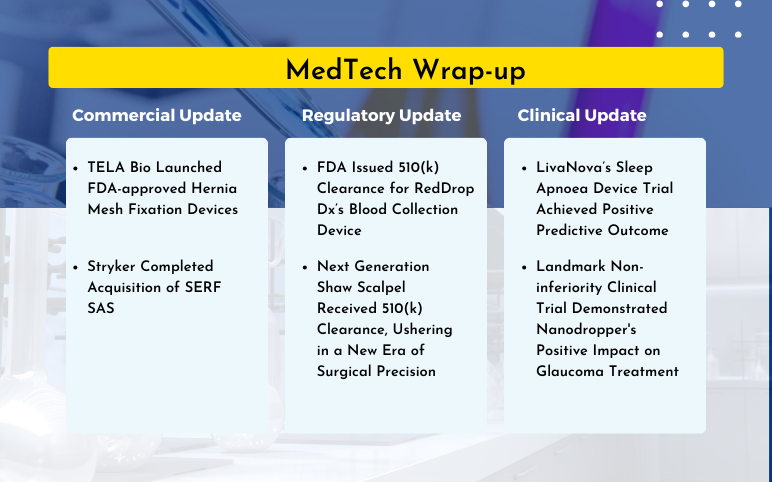
TELA Bio Launched Hernia Mesh Fixation Devices; Stryker Acquisition of SERF SAS; FDA 510(k) Clearance for RedDrop Dx’s Blood Collection Device; FDA 510(k) Clearance for Next Generation Shaw Scalpel; LivaNova’s Sleep Apnoea Device Trial; Nanodropper’s Positive Impact on Glaucoma Treatment
TELA Bio Launched FDA-approved Hernia Mesh Fixation Devices On March 21, 2024, TELA Bio, Inc., a medical technology firm, launched its FDA-approved LIQUIFIX devices for hernia repairs in the United States. The LIQUIFIX FIX8™ and LIQUIFIX Precision™ are specifically engineered for laparoscopic and open femo...
Read More...
Mar 26, 2024
Regeneron’s Odronextamab BLA; Novo Nordisk’s Cardior Pharmaceuticals Acquisition; Novartis’ Fabhalta CHMP Approval; Idorsia’s TRYVIO FDA Approval; AbbVie’s Landos Biopharma Acquisition
Regeneron Updates Progress on Biologics License Application for Odronextamab Regeneron Pharmaceuticals, Inc. has announced that the FDA has issued Complete Response Letters (CRLs) regarding the Biologics License Application (BLA) for odronextamab in cases of relapsed/refractory (R/R) follicular lymphoma (FL) and...
Read More...
Mar 21, 2024
Vygon Launched Nutrisafe2 System; Butterfly Network Launched Third-Gen Ultrasound; Cosm Medical Won FDA Clearance; FDA Cleared Sequel Med Tech Automated Insulin Delivery System; IceCure Medical Reported Positive Topline Results; Cerus Announced Positive Results in Blood Purification Trial
Vygon Launched Neonatal Safety in India with Groundbreaking Nutrisafe2 System On March 15, 2024, Vygon, a leader in the production and marketing of specialized medical equipment, introduced Nutrisafe2, a technological leap forward. This state-of-the-art enteral feeding device for neonates guarantees the sa...
Read More...
Mar 14, 2024
Allumiqs and Prolytix’s Partnership; Setpoint Medical’s Neuroimmune Modulation Platform gets FDA Breakthrough Designation; MangoRx Launches ‘PRIME’ with FDA-approved TRT; SeaStar Medical Updates Quelimmune Commercial Launch; Ventris Medical Gains 510(k) for Amplify® Bone Graft Putty
MangoRx Officially Launches ‘PRIME’ by MangoRx, Powered by Kyzatrex®️ FDA Approved Oral Testosterone Replacement Therapy (TRT) Treatment On March 12, 2024, Mangoceuticals, Inc. unveiled a groundbreaking development eagerly awaited by many: the launch of 'PRIME' by MangoRx, Powered by Kyzatrex®️. This release mar...
Read More...







-Agonist.png)

