Fruquintinib
Apr 04, 2023
HUTCHMED’s NDA Submission to FDA for Fruquintinib; Cytokinetics to Discontinue ALS Drug Candidate Following Phase III Trial Failure; Enfortumab Vedotin + Pembrolizumab Approved for Urothelial Carcinoma; Nanoscope Gene Therapy Clears Phase II Retinitis Pigmentosa Trial; FDA Clearance to Cabaletta Bio’s IND Application for CABA-201 for SLE Treatment; European Orphan Drug Designation to Gene Therapy Candidate DB-OTO
HUTCHMED Completes Rolling Submission of NDA to FDA for Fruquintinib HUTCHMED (China) Limited announced the completion of the rolling submission of a New Drug Application ("NDA") to the United States Food and Drug Administration ("FDA") for fruquintinib, its highly selective and potent oral inhibitor of VEGFR-1,...
Read More...
Mar 06, 2017
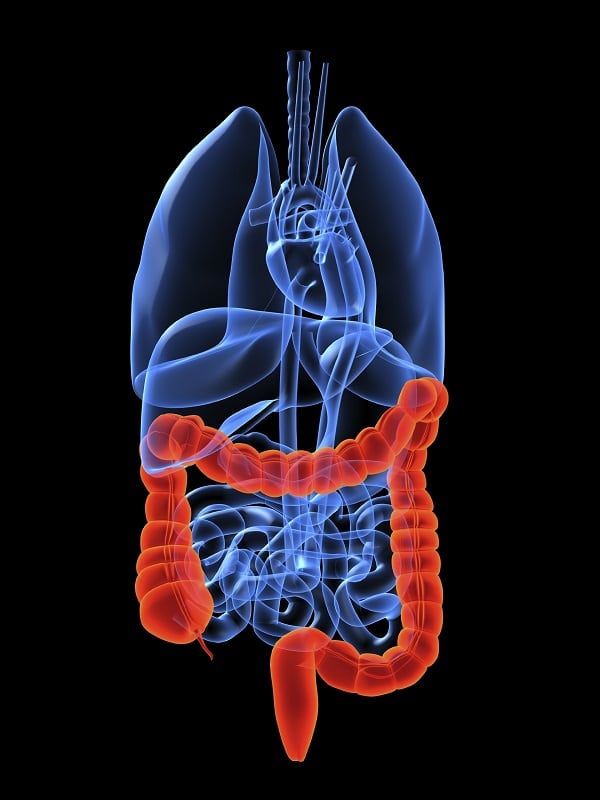
Colorectal Cancer Market Analysis and Market Forecast
Colorectal Cancer is a formidable health problem worldwide which is the third most common cancer diagnosed among men and women. It arises as a polyp which later develops into a life-threatening cancer; it occurs due to the unrestrained cell growth in appendix, colon, and the rectum region. High incidence rate is obs...
Read More...

-Agonist.png)

