Gene therapy
Nov 25, 2019
How Gene therapy is changing the Beta-thalassemia Treatment outlook?
Beta thalassemia, globally affects almost 288,000 people, with an incidence of 60,000 cases every year. A rare monogenic genetic disease, beta-thalassemia is caused by more than 200 mutations of the beta-globin gene (HBB). The Beta-thalassemia pharmacological treatment mainly comprises of regular blood-transfus...
Read More...
May 27, 2019
Meet the World’s most expensive drug: Zolgensma
The U.S. FDA recently approved the much talked about Novartis’s drug Zolgensma for treating a life-threatening condition Spinal Muscular Atrophy (SMA). SMA is a neuromuscular disorder that worsens the muscles over time. It happens due to a mutation in the SMN1 gene that results in the deficiency of an SMN pro...
Read More...
Apr 19, 2019
Can commoners afford to spend million dollars on GENE Therapy?
Each country has a unique system, politics, and economy. Different policies are designed to suit people of different countries. Same goes with the Healthcare industry. Healthcare is financed either through government voluntary or compulsory insurances or via private corporations and NGOs. Industrialised and more d...
Read More...
Apr 10, 2019
The promising Pipeline for Gene Therapy In Oncology
Gene therapy is a technique that involves replacing, inactivating, or adding a new gene to treat or prevent a disease. The science of GENE THERAPY involves the DNA-based changes in genetic material so that the gene can transcribe a healthy protein. In the past, there has been no success in designing any gene therap...
Read More...
Mar 21, 2019
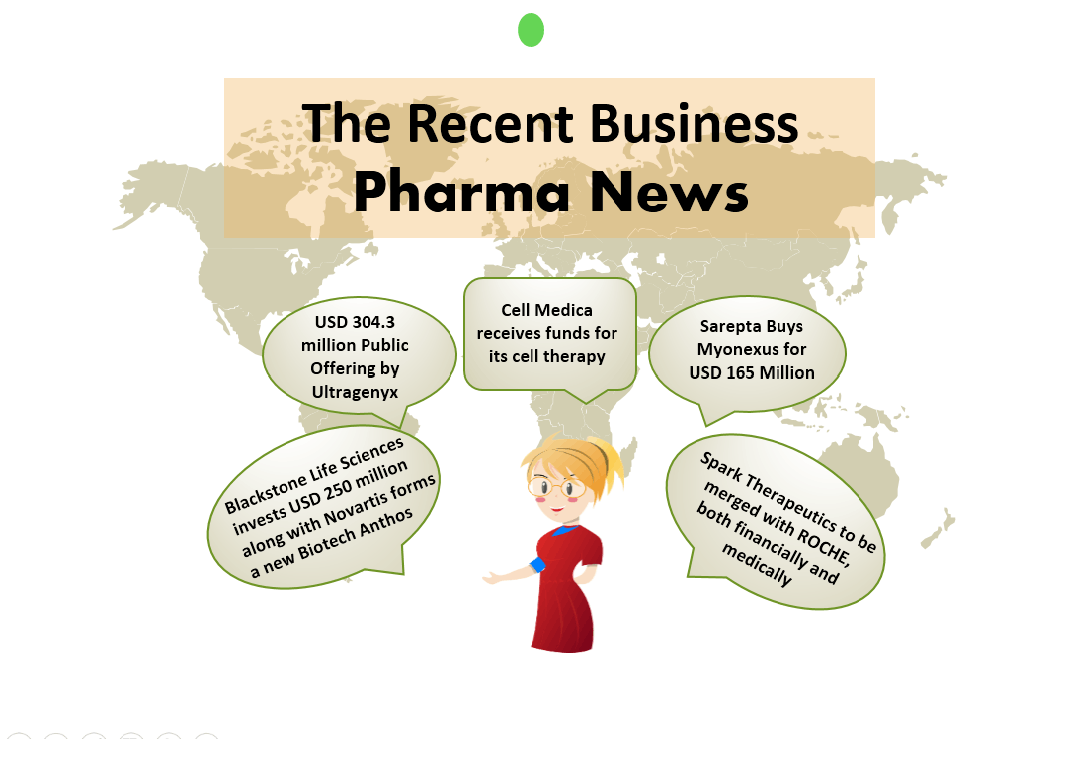
The Business Cocktail
Biohaven buys PRV from GW Pharmaceuticals for USD 105 million GW Pharmaceuticals has announced its decision to sell its rare pediatric disease priority review voucher (PRV) to Biohaven Pharmaceuticals. The voucher was issued under U.S. Food and Drug Administration programme to accelerate speedy recovery of rare ...
Read More...
Feb 28, 2019
The Business Cocktail
Cell Medica receives funds for its cell therapy A USD 8.7 million grant to Cell Medica has been given by Cancer Prevention and Research Institute of Texas (CPRIT) to speed up the clinical development of CMD-502 off-the-shelf chimeric antigen receptor-natural killer T cell (CAR-NKT) cell therapy. The program, su...
Read More...
Dec 27, 2018
Vedanta raises; Relay Therapeutics reels in; Gene therapy for SMA
Vedanta raises USD 27 Million to move microbiome assets through the clinic Vedanta Biosciences raises USD 27 million in its series C round. The cash will bolster its microbiome-derived drugs pipeline. The capital will aid a phase 1/2 trial of VE416 in food allergy, a phase 2 study of VE303 in recurrent...
Read More...
Oct 12, 2018
Is Gene Therapy the Next Cancer Treatment Revolution?
Gene Therapy is now touted as a new revolution for cancer treatment. If the list of new cancer treatment methodologies is traversed, then the end seems to be farther and farther away. As a sequel to the relentless efforts by researchers across the world to formulate therapies better than the existing ones, new prosp...
Read More...
Aug 31, 2018
AbbVie Receives Approval; Kymriah Approved; Shire Gets USFDA Approval; NICE Rejects; Bristol-Myers Squibb’s Opdivo; Eisai and Merck Get USFDA Approval
AbbVie Receives USFDA Approval for Imbruvica Plus Rituximab as First Chemotherapy-Free Combination Treatment for Waldenström's Macroglobulinemia Adults AbbVie announced that the USFDA approved Imbruvica (ibrutinib) plus rituximab (Rituxan) for the treatment of adult patients with Waldenström's macroglobulinemia (W...
Read More...
Aug 29, 2018
Snippet
National health authorities, U.K.'s NICE determines Gilead's cancer cell therapy exorbitant The National Institute for Health and Care Excellence (NICE) found Gilead's cancer cell therapy, Yescarta to be clinically efficacious for treating a type of lymphoma, but no ample evidence is present to estimate the benefit...
Read More...





-Agonist.png)

