glaucoma
Mar 27, 2025
Alcon Gains CE Mark for Clareon Vivity IOL in Europe; MicroPort MedBot’s Toumai SP Robot Wins NMPA Approval; Abbott Launches Intravascular Lithotripsy Trial; BD Advances GalaFLEX LITE™ Clinical Trial; GE HealthCare Unveils AI-Powered Invenia ABUS Premium; Okami Medical Introduces SENDERO® MAX Catheter
Alcon Announced CE Mark Approval and Launch of Clareon Vivity IOL in Europe, Expanding Visual Options On March 25, 2025, Alcon, the global leader in eye care committed to enhancing vision, announced that Vivity®, the most widely implanted extended depth of focus (EDOF) intraocular lens (IOL), became availa...
Read More...
Apr 12, 2024
The Unmet Needs in Glaucoma Treatment: Bridging Gaps for Better Vision Care
Glaucoma, often dubbed as the “silent thief of sight,” poses a significant challenge in the domain of ophthalmology. Glaucoma treatment has made significant strides, yet several unmet needs persist, posing challenges to effective management and vision preservation. By prioritizing early detection, embracing persona...
Read More...
Jul 06, 2023
Enovis Acquires Novastep; Rockwell Medical Announced Collaboration with B. Braun; Dexcom G7 Received Health Canada Approval for Next-Generation CGM; Abbott’s World’s First Dual Chamber Leadless Pacemaker; Elios Vision’s Pivotal Trial of ELIOS; Synergy Spine’s Synergy Disc 2-Level IDE Trial
Enovis completed the acquisition of Novastep On June 29, 2023, Enovis, one of the largest orthopedic device companies in the world, announced that the company had completed the purchase of Novastep and its foot and ankle minimally invasive surgical (MIS) platform, which was first announced in April 2023. ...
Read More...
Jan 05, 2023
Inspira and Terumo Signed Strategic Agreement; Cook Medical’s New Bipolar Electrodes Portfolio; Verana Health and Sight Sciences Collaborated on Glaucoma Research; Burning Rock’s OverC Multi-Cancer Detection Blood Test; Innova Vascular’s Thrombectomy System; liberDi’s Digital Dialysis System
Inspira Technologies Signed Strategic OEM Agreement with Terumo Cardiovascular On December 27, 2023, Inspira Technologies, a ground breaking respiratory support technology company, announced that it has signed an exclusive OEM (Original Equipment Manufacturing) agreement with Terumo Cardiovascular, a division of...
Read More...
Jan 04, 2023
Insights Into the Evolving Landscape of the Ocular Implants Market
Vision loss or vision impairment is one of the major issues globally that can affect people of all ages. As per the WHO, Globally, about 2.2 billion people have a near or distance vision impairment. Moreover, in almost half of the cases, vision impairment could have been prevented or has yet to be addressed. It is ...
Read More...
Sep 27, 2022
Daiichi Sankyo’s Ezharmia; Pfizer & Sangamo Hemophilia A Gene Therapy Trial; Approval for Fennec’s Pedmark; FDA Approves UBE and Santen’s OMLONTI; EC Approves AstraZeneca’s Tezspire; FDA Approves Selpercatinib; FDA Grants Accelerated Approval to Eli Lilly’s Retevmo; GSK & Spero Announce Exclusive License Agreement
Daiichi Sankyo Receives the First Approval for its Blood Cancer Drug Ezharmia Daiichi Sankyo has received the first global approval for Ezharmia, a first-in-class dual EZH1 and EZH2 inhibitor for the treatment of patients with relapsed or refractory adult T-cell leukemia/lymphoma (ATL). The Japanese Ministry of ...
Read More...
Aug 11, 2022
Rapid Medical’s TIGERTRIEVER 13; Glaukos’s Istent Infinite System; GE Healthcare’s Definium 656 HD; NeuroOne’s Signed Exclusive Development & Distribution Agreement with Zimmer; Kaia Health’s Rise-uP Randomized Controlled Trial; Bluejay’s Symphony IL-6 Test
Rapid Medical Obtains FDA Clearance for the World's Smallest and Only Adjustable Thrombectomy Device On July 26, 2022, Rapid Medical, a leading developer of advanced neurovascular devices, received Food and Drug Administration (FDA) 510(k) clearance for TIGERTRIEVER™13 for large vessel occlusions.&n...
Read More...
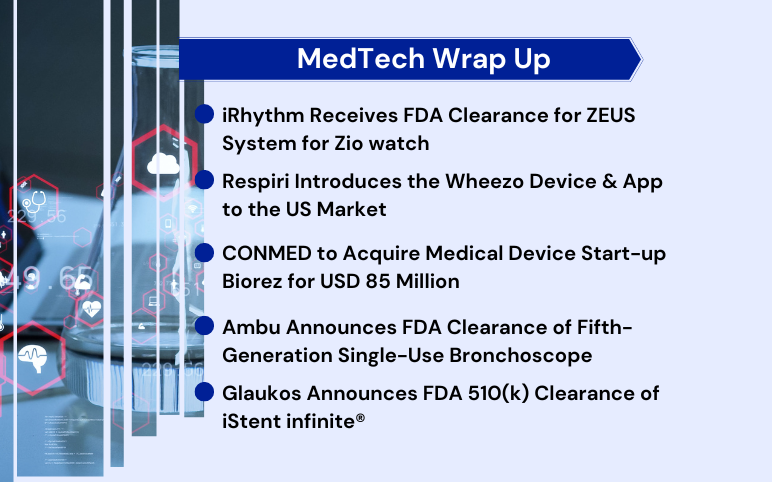
Aug 04, 2022
FDA Clearance to iRhythm’s ZEUS System; Respiri Introduces the Wheezo Device & App; CONMED to Acquire Biorez for $ 85 Million; FDA Clearance to Ambu’s Single-Use Bronchoscope; FDA 510(k) Clearance to Glaukos’s iStent infinite
iRhythm receives FDA clearance for ZEUS System for Zio watch iRhythm Technologies, a leading digital healthcare solutions firm focused on advancing cardiac care, announced that it received FDA 510(k) clearance for the ZEUS (Zio ECG Utilization Software) System for the Zio Watch. It is produced in partnership wit...
Read More...
Apr 18, 2022
Global Top Players in Intraocular Lens (IOL) Market
An Intraocular Lens implant is a synthetic, artificial lens placed inside the eye that replaces the focusing power of a natural lens that is surgically removed, usually as part of cataract surgery. Most Intraocular Lenses (IOL) are made of silicone, acrylic, or other plastic compositions and are also coated w...
Read More...
Apr 15, 2022
Prominent Players in Glaucoma Drainage Devices Market
Glaucoma is a group of progressive optic neuropathy which is characterized by the degradation of ganglion cells present in the retina. The loss of the retinal ganglion is associated with intraocular pressure (IOP), an elevated IOP due to an imbalance in the production and drainage of aqueous humor that might lead t...
Read More...








-Agonist.png)

