Hip Replacement Devices
Dec 05, 2024
FDA Approves Zimmer Biomet’s Oxford® Cementless Partial Knee; ReCerf® Sets Milestone as First All-Ceramic Hip Resurfacing Approved Worldwide; Orthocell Achieves US FDA 510(k) Clearance Milestone for Remplir Regulatory Study; Hologic’s Research Shows AI Breast Imaging Maintains Performance Across All Demographics; GE HealthCare Expands MRI Capabilities with Sonic DL’s Advanced Deep Learning; Terumo Unveils the R2P NaviCross Peripheral Support Catheter
Zimmer Biomet Received FDA Approval for Oxford® Cementless Partial Knee, the Only Cementless Partial Knee Replacement Implant in the U.S. On November 25, 2024, Zimmer Biomet Holdings, Inc., a global leader in medical technology, announced that its Oxford® Cementless Partial Knee received Premarket Approval...
Read More...
May 16, 2024
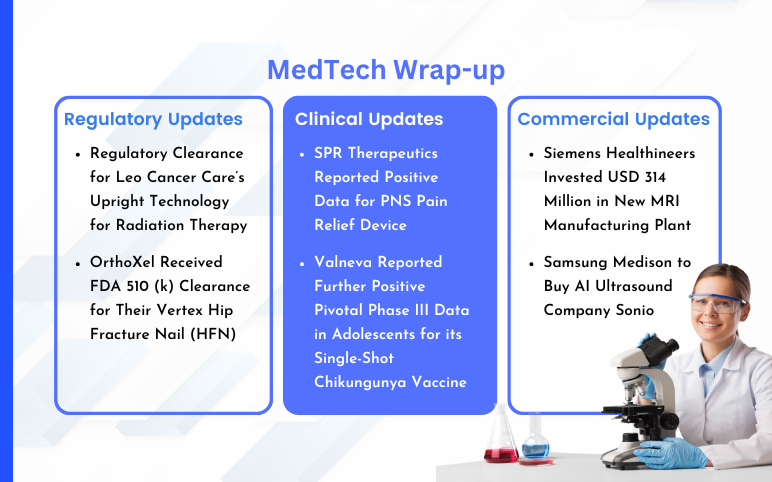
Siemens Healthineers USD 314 Million Investment; Samsung Medison’s Sonio Acquisition; Leo Cancer Care’s Upright Technology Clearance; OrthoXel’s Vertex Hip Fracture Nail FDA 510 (k) Clearance; SPR Therapeutics’ PNS Pain Relief Device Positive Data; Valneva’s Chikungunya Vaccine Positive Pivotal Phase III Data
Siemens Healthineers Invested USD 314 Million in New MRI Manufacturing Plant On May 15, 2024, Siemens Healthineers announced that it had invested USD 314 million in a new MRI manufacturing plant in the UK. The facility in Oxford, England, which Siemens anticipates opening in 2026, will develop technology to redu...
Read More...
Jul 21, 2022
FDA Approval to Centinel’s Total Disc Replacement Devices; FDA clears DyAnsys’ Neurostimulator; Orthox to Commence FFLEX Study; Cordis Started Enrolment for RADIANCY Clinical Study; Orthofix & LimaCorporate Announce Partnership; Medtronic to Partner With AWS
Orthox Obtains MHRA Approval to Commence FFLEX Study On July 14, 2022, Orthodox Ltd, a clinical-stage company created medical implants to treat orthopedic problems including damaged knee articular cartilage. It was founded in 2008 by life scientist, Dr. Nick Skaer, and knee surgeon, Professor Oliver Kessle...
Read More...


-Agonist.png)

