Interstitial lung disease
Aug 20, 2024
Gilead’s Livdelzi FDA Approval for Primary Biliary Cholangitis; Incyte and Syndax’s Niktimvo Approved for Graft-Versus-Host Disease; FDA Lifts Hold on BioNTech and MediLink’s ADC Cancer Trial; FDA Approves IMFINZI for Resectable Lung Cancer; Yutrepia Receives Tentative Approval for PAH and PH-ILD
Gilead’s Livdelzi (Seladelpar) Granted Accelerated Approval for Primary Biliary Cholangitis by FDA Gilead Sciences, Inc. has received accelerated approval from the FDA for Livdelzi® (seladelpar) in the treatment of primary biliary cholangitis (PBC). Livdelzi can be used in combination with ursodeoxycholic acid (...
Read More...
Aug 10, 2020
Boehringer Ingelheim; Roche demonstrated new analyses of their drugs in patients with ILDs during the American Thoracic Society (ATS) Virtual conference
ATS 2020 Virtual is scheduled to be held from August 5 to August 10, and it is exciting to watch the key presentations by Pharmaceutical Companies specifically focused on pulmonary disease, critical illness, and sleep disorders. Boehringer Ingelheim presented new analyses of Ofev data in patients with chronic fi...
Read More...
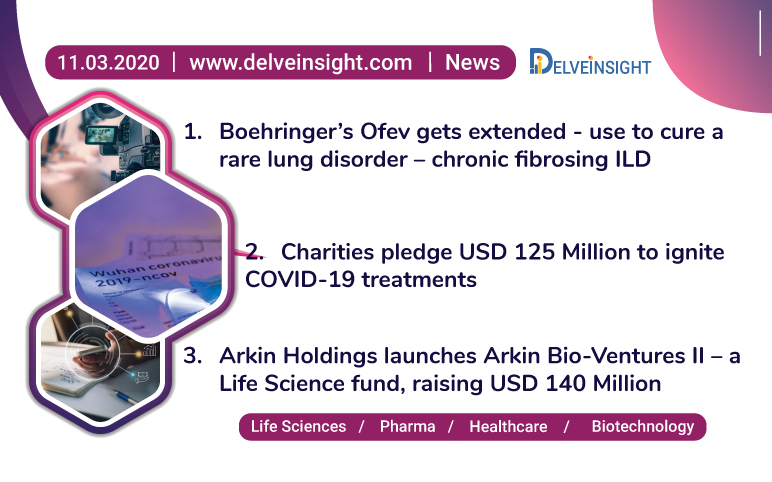
Mar 11, 2020
Ofev’s expanded use; Arkin Bio-Ventures II launch; USD 125 M for COVID-19 treatment
Boehringer Ingelheim’s Ofev (nintedanib) has received FDA recommendation for the treatment of chronic fibrosing interstitial lung diseases (ILD) with a progressive phenotype Interstitial Lung Disease - ILD – a group of a large number of lung disorders resulting in scarring or fibrosis of lungs, caused by conditi...
Read More...

-Agonist.png)

