Ipsen
Feb 23, 2024
Ipsen’s Onivyde Combo Ends a Decade-Long Dry Spell in Newly Diagnosed Pancreatic Cancer
It marks the awaited green light for Ipsen following its acquisition of Onivyde in 2017. The FDA has given its nod to the supplemental new drug application for Onivyde (irinotecan liposome injection) in combination with oxaliplatin, fluorouracil, and leucovorin (NALIRIFOX) as a primary treatment for adults with met...
Read More...
Dec 12, 2023
Merck and Moderna Initiate Study to Evaluate V940; FDA Approves Vertex and CRISPR Therapeutics’ CASGEVY for SCD; Novartis Updated on its Investigational Iptacopan Phase III Study; FDA Grants Priority Review for New Drug Application for Elafibranor; FDA Approves bluebird bio’s LYFGENIA for Patients SCD; FDA Fast Track Designation for DMD Gene Therapy
Merck and Moderna Initiate INTerpath-002, a Phase III Study Evaluating V940 in Combination with KEYTRUDA for Adjuvant Treatment of Patients with Certain Types of Resected NSCLC Merck (also known as MSD outside the United States and Canada) and Moderna, Inc. have commenced the INTerpath-002 trial—a crucial Phase ...
Read More...
Jun 20, 2023
FDA Extended the Review Period for Momelotinib; FDA Approves Roche’s Columvi; Avita Medical Obtains FDA Approval of RECELL; FDA Approves Odevixibat for ALGS; TME Pharma Receives IND Approval for NOX-A12; Aldeyra’s Phase 3 INVIGORATE‑2 Trial of Reproxalap
GSK Announces Extension of FDA Review Period for Momelotinib After all, GSK will not hear from the FDA this month about its marketing application for momelotinib as a therapy for anemia in myelofibrosis patients. The pharmaceutical company announced that the US Food and Drug Administration has extended the drug'...
Read More...
Apr 11, 2023
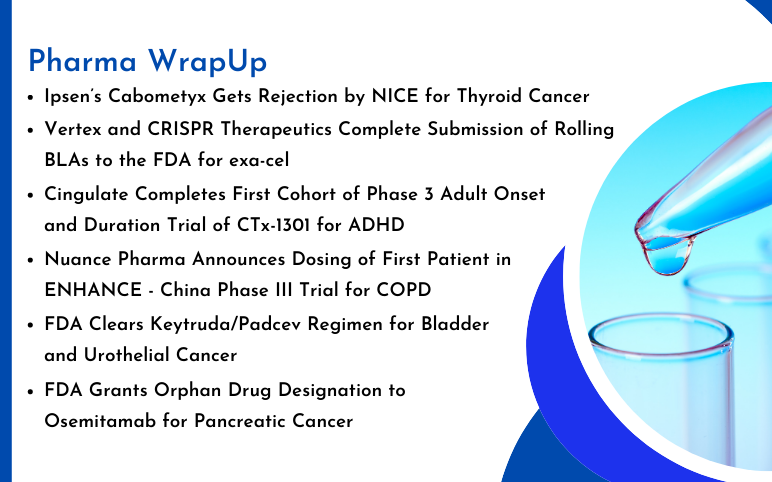
Ipsen’s Cabometyx Rejected by NICE; Vertex and CRISPR Therapeutics’s Submit BLA to the FDA for exa-cel; Orphan Drug Designation to Osemitamab for Pancreatic Cancer; FDA Clears Keytruda/Padcev for Bladder and Urothelial Cancer; Cingulate Completes Trial of CTx-1301 for ADHD; Nuance Pharma Announces Dosing of First Patient in ENHANCE Trial
FDA Grants Orphan Drug Designation to Osemitamab for Pancreatic Cancer Transcenta Holding Limited has announced that the U.S. Food and Drug Administration (FDA) has awarded Orphan Drug Designation to Osemitamab (TST001), a highly potent humanized monoclonal antibody that enhances ADCC (antibody-dependent cell-me...
Read More...
Jan 10, 2023
Ipsen to Acquire Albireo; Chiesi Farmaceutici to Buy Amryt Pharma; Takeda Presents Phase III Results of TAK-755 for cTTP; ACELYRIN Acquires ValenzaBio; FDA Approves LEQEMBI for Alzheimer’s Disease; Orphan Drug Designation to Lantern Pharma’s LP-284 for MLL
Ipsen to Acquire Albireo for USD 952 Million Ipsen, a French pharmaceutical company, has offered USD 42 per share for the US biotech Albireo and its rare disease treatment Bylvay, continuing a recent spree of merger and acquisition activity. Albireo, a Boston-based business, is valued at USD 952 million under th...
Read More...
Oct 17, 2019
EicOsis receives USD 15M to fuel its non-opioid pain therapy; Alexion Pharmaceuticals buys Achillion Pharmaceuticals; Ipsen adds another drug to its rare disease pipeline
EicOsis has received USD 15Million grant from the National Institute of Drug Abuse (NIDA) to fund the clinical trials of non-opioid pain therapy. NIDA, with an aim to supplement NIH’s initiative Helping to End Addiction Long-Term (HEAL Initiative), has awarded the grant to the company. Deaths due to drug ov...
Read More...
Jan 12, 2017
Aurobindo takes top generic spot; Valeant unloads Dendreon; Ipsen strikes $1B deal; Takeda shells out $5.2B
Aurobindo takes top generic spot in Portugal with €135M deal for Generis Farmacêutica India’s Aurobindo Pharma, which has been building its presence around the world, has struck a new deal to buy a generics manufacturer and plant in Portugal which it says will make it the largest generics manufacturer in that countr...
Read More...





-Agonist.png)

